FIGURE 7.
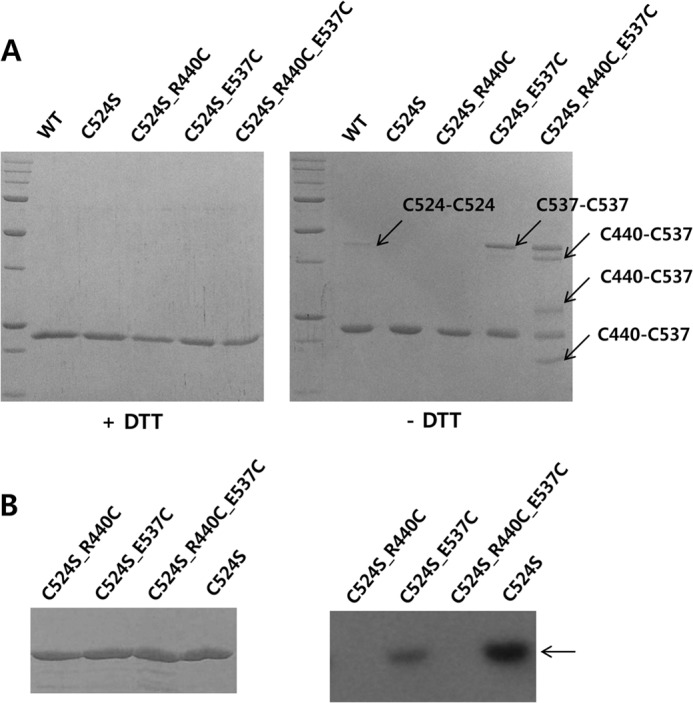
Arg440 and Glu537 can be in close proximity in the KC and these residues are essential for autophosphorylation. A, mutation of Arg440 and Glu537 to cysteines induced disulfide bond formation between the two residues. Although wild-type KC and four mutants (C524S, C524S/R440C, C524S/E537C, and C524S/R440C/E537C) were shown as a single band by SDS-PAGE in the presence of dithiothreitol, dimer-sized bands were detected in WT, C524S/E537C, and C524S/R440C/E537C in the absence of the reducing agent. The presence of multiple bands in the C524S/R440C/E537C mutant indicates various interactions between Cys440 and Cys537 in a KC dimer. B, mutation of Arg440 or Glu537 leads to a loss of the autophosphorylation activity compared the C524S mutant; activity of the R440C mutant was completely ablated.
