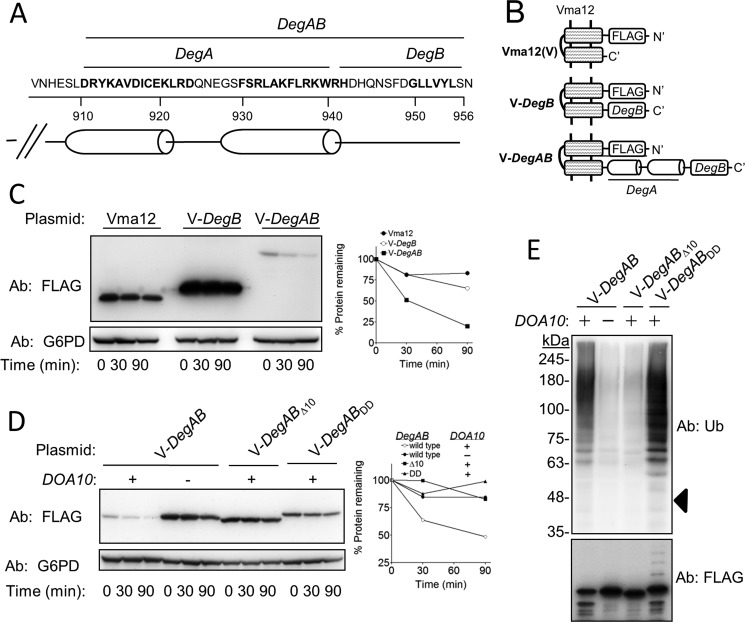
FIGURE 1.
The hydrophobic core of DegB of the Ndc10 degron is dispensable for ubiquitylation but essential for proteasomal degradation. A, amino acid sequence and structure prediction of the Ndc10 degron are shown. B, schematic presentation of Vma12-degron fusions used in C–E. V-DegB: Vma12-DegB; V-DegAB: Vma12-DegAB. C, DegB is insufficient to confer degradation to Vma12. Cells expressing the indicated proteins were subjected to cycloheximide-chase degradation assay, as described under “Experimental Procedures.” D, intact DegB is required for the degradation of Vma12-DegAB. Wild type and doa10Δ cells, expressing intact Vma12-DegAB or a truncated protein in which the last 10 aa were removed (Δ10), or a mutant protein in which two Leu residues at the hydrophobic core of DegB were replaced with Asp (DD), were subjected to cycloheximide degradation assay. E, the DD mutation within DegB did not impair the ubiquitylation of Vma12-DegAB. The various Vma12-DegAB proteins described in D were purified from cells using anti FLAG-agarose affinity gel. PolyUb chains conjugated to V12-DegAB were detected after separation on 5–15% gradient SDS-PAGE. Triangle indicates the estimated molecular mass of unconjugated V-DegAB.