FIGURE 1.
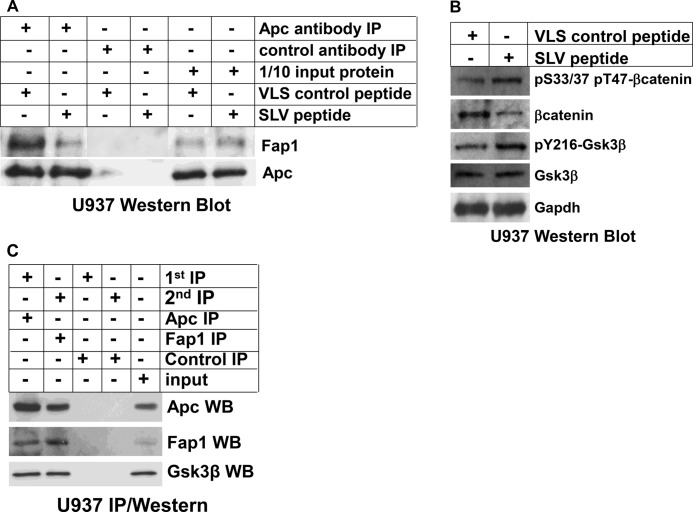
Fap1 influences Gsk3β activity, βcatenin protein expression, and serine/threonine phosphorylation of βcatenin in myeloid cell lines. A, treatment of U937 myeloid leukemia cells with SLV peptide disrupts interaction between Fap1 and Fas. U937 cells were treated with Fap1-blocking SLV peptide or control VLS peptide. Cell lysates were immunoprecipitated (IP) under non-denaturing conditions with an antibody to Apc or irrelevant control antibody. Immunoprecipitates were analyzed by Western blot analysis for coprecipitation of Fap1 with Apc. Non-immunoprecipitated lysates were analyzed as a loading control. B, treatment with of U937 myeloid leukemia cells with SLV peptide increases tyrosine phosphorylation of Gsk3β, decreases total βcatenin protein, and increases serine/threonine phosphorylated βcatenin. U937 cells were treated with Fap1-blocking SLV peptide or control VLS peptide. Cell lysates were analyzed by Western blot analyses serially probed with antibodies to total βcatenin, βcatenin phosphorylated on Ser-33/37 or Thr-47, Gsk3β, Gsk3β phosphorylated on Tyr-216, or Gapdh (as a loading control). C, Fap1, Apc, and Gsk3β are present in a single protein complex. U937 cell lysate proteins were immunoprecipitated under non-denaturing conditions with an Apc antibody or irrelevant control antibody. Immunoprecipitates were recovered, and a second round of immunoprecipitation was performed with a Fap1 antibody or irrelevant control antibody. Immunoprecipitates were separated by SDS-PAGE, and Western blot analyses (WB) were probed with antibodies to Apc, Fap1, and Gsk3β. Non-precipitated proteins were used as a loading control.