FIGURE 6.
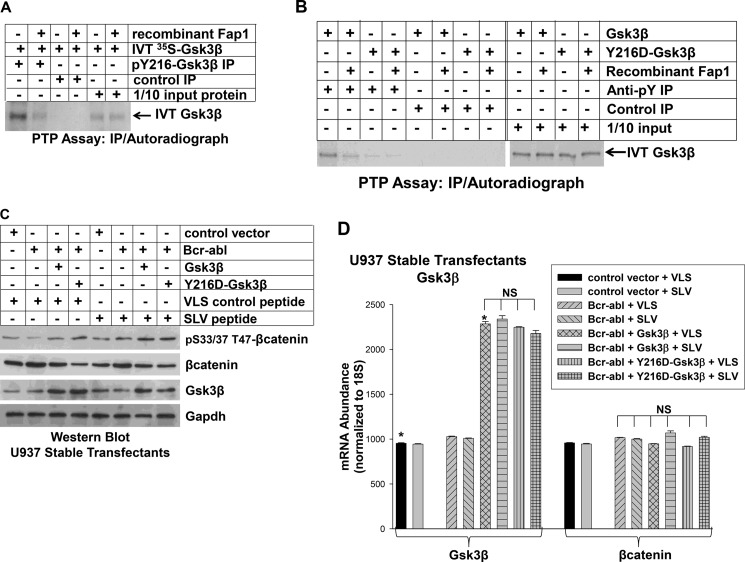
Fap1 decreases activity and tyrosine phosphorylation of Gsk3β. A, recombinant Fap1 dephosphorylates in vitro-translated (IVT) Gsk3β. Gsk3β was translated in vitro in rabbit reticulocyte lysate (in the presence of [35S]methionine) and incubated with recombinant Fap1 or with buffer control. Proteins were immunoprecipitated (IP) with an antibody to phospho-Tyr-216-Gsk3β or irrelevant control antibody, separated by SDS-PAGE, and identified by autoradiography. 1/10 input (non-immunoprecipitated) Gsk3β was used as a loading control. B, recombinant Fap1 dephosphorylates Gsk3β primarily at Tyr-216. Gsk3β or a form of Gsk3β with mutation of Tyr-216 (Y216D) were in vitro-translated in rabbit reticulocyte lysate as above. Proteins were incubated with recombinant Fap1 or buffer control and immunoprecipitated with an antibody to phospho-tyrosine residues (4G10) or with irrelevant control antibody. Immunoprecipitates were separated by SDS-PAGE and detected by autoradiography. C, expression of constitutively active Gsk3β (Y216D) in Bcr-abl+ U937 cells decreases βcatenin protein and increases serine/threonine phosphorylation of βcatenin in a manner that is not influenced by SLV peptide. U937 cells were cotransfected with a Bcr-abl expression vector and a vector to express Y216D-Gsk3β or WT Gsk3β (or empty control vector). Cells were treated with Fap1-blocking SLV peptide or VLS control peptide and lysates and analyzed by Western blot analysis. Blots were serially probed with antibodies to total and serine/threonine phosphorylated βcatenin, Gsk3β, and Gapdh (as a loading control). D, expression of constitutively active Gsk3β (Y216D) in Bcr-abl+ U937 cells does not alter expression of βcatenin mRNA. The stably transfected U937 cells described above were also analyzed for mRNA expression of Gsk3β and βcatenin by real-time PCR. Statistically significant differences are indicated by *. NS, not significant.