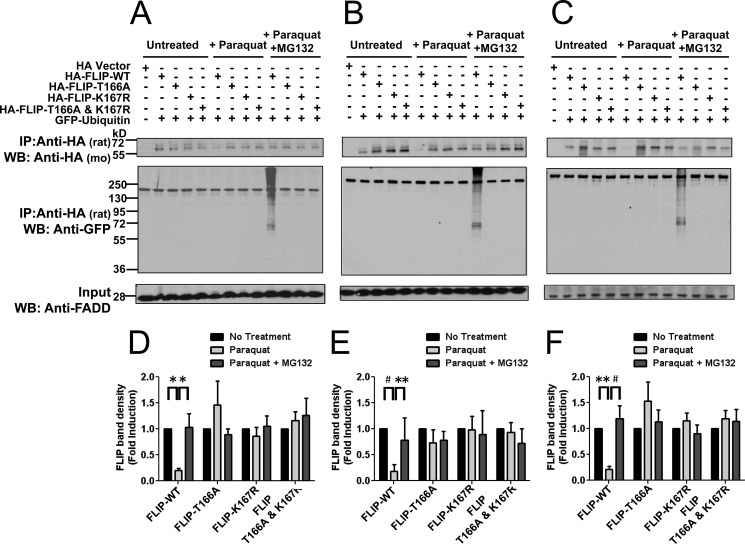
FIGURE 5.
Phosphorylation of Thr-166 and ubiquitination of Lys-167 are required for ROS-dependent ubiquitination and degradation of FLIP following paraquat treatment. A-C, PPC1 (A), HeLa (B), or 293T (C) cells were co-transfected with EGFP-C2-ubiquitin and either pcDNA3-HA vector (first lane) or various HA-tagged FLIP plasmids (WT, T166A, K167R, and T166A,K167R double mutant) for 16 h. Cells were then treated with paraquat (2 mm) in the presence of MG132 (0.5 μm) for 8 h. Cell lysates were immunoprecipitated (IP) using rat anti-HA antibody and the immunoprecipitated proteins were analyzed by immunoblotting using mouse anti-HA to detect FLIP and anti-GFP to detect ubiquitination. The inputs (1/10 of lysates used for immunoprecipitation) were analyzed by immunoblotting using rabbit anti-FADD antibody as a loading control. D–F, levels of HA-FLIP protein in A–C, respectively, were quantified using scanning densitometry. Vector groups with no treatment were adjusted to 1. Statistical significance (mean ± S.E.; n = 6 PPC1 and 293T; n = 4 HeLa) was determined by two-way analysis of variance and Bonferroni post-test. *, indicates the p value is p < 0.05; **, indicates p < 0.01; #, indicates p < 0.001.