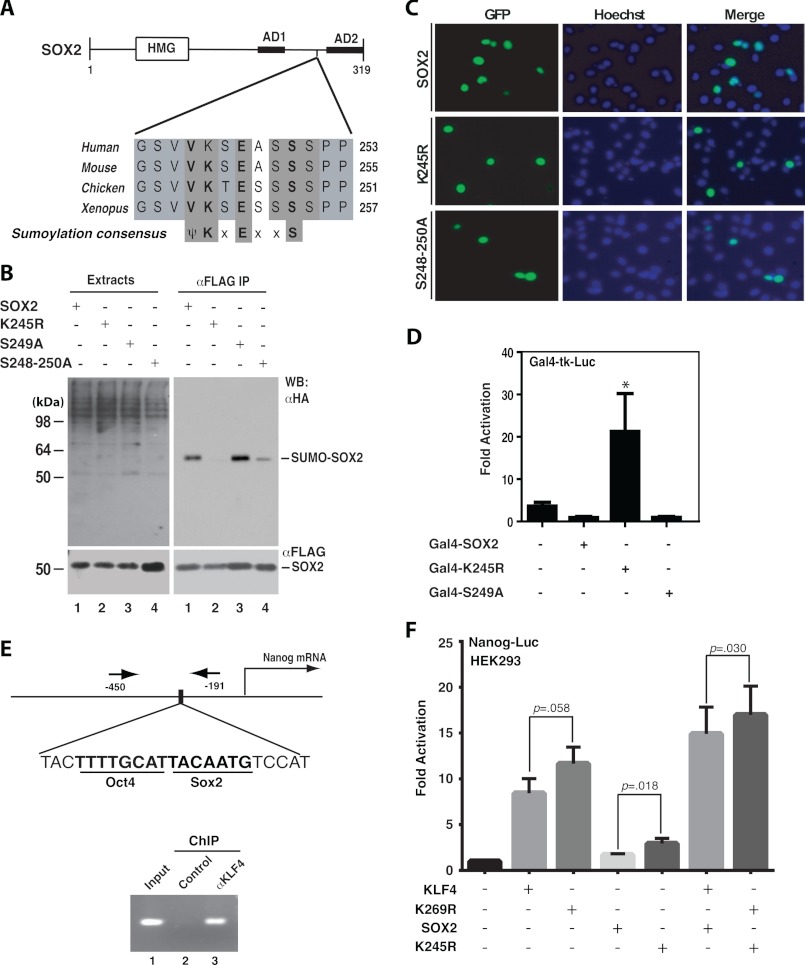
FIGURE 2.
Sumoylation inhibits transcriptional activity of SOX2. A, domain organization of mouse Sox2 shown with sequence alignment of a conserved sumoylation motif and adjacent residues in Sox2 proteins from Xenopus to humans. HMG, high mobility group DNA binding domain; AD1 and AD2, transcriptional activation domains 1 and 2, respectively. B, sumoylation assays. Expression plasmids for HA-SUMO2 and FLAG-tagged wild-type human SOX2 and mutants were co-transfected into HEK293 cells. Extracts were prepared as in Fig. 1B for IP on anti-FLAG M2-agarose and subsequent immunoblotting with anti-HA and -FLAG antibodies as indicated. C, subcellular localization of GFP-tagged wild-type and mutant SOX2 proteins. After transient transfection with the corresponding expression plasmids, HEK293 cells were fixed and incubated with Hoechst 33258 for fluorescence microscopy to detect GFP expression (green) and nuclei (blue). D, reporter gene assays. The assays were performed as for Fig. 1D except that expression plasmids for Sox2 and mutants were analyzed. The results were calculated from three independent assays. *, p < 0.05 when compared with the empty vector-transfected control. E, ChIP analysis. Formaldehyde was used to fix mouse ES cells, and after brief sonication, soluble chromatin was prepared for IP with the α-KLF4 antibody (lane 3) or control IgG (lane 2). The immunoprecipitates were used for PCR to amplify the fragment corresponding to nucleotides −450 to −191 of the mouse Nanog promoter, indicated by arrows (top). F, reporter gene assays. The assays were performed as for Fig. 1D except that the reporter construct contains a mouse Nanog promoter fragment (−2340 to +150) controlling luciferase expression. The expression plasmids for FLAG-tagged KLF4 and SOX2 were transfected as specified. The results were calculated from five different sets of data, with p values indicated.