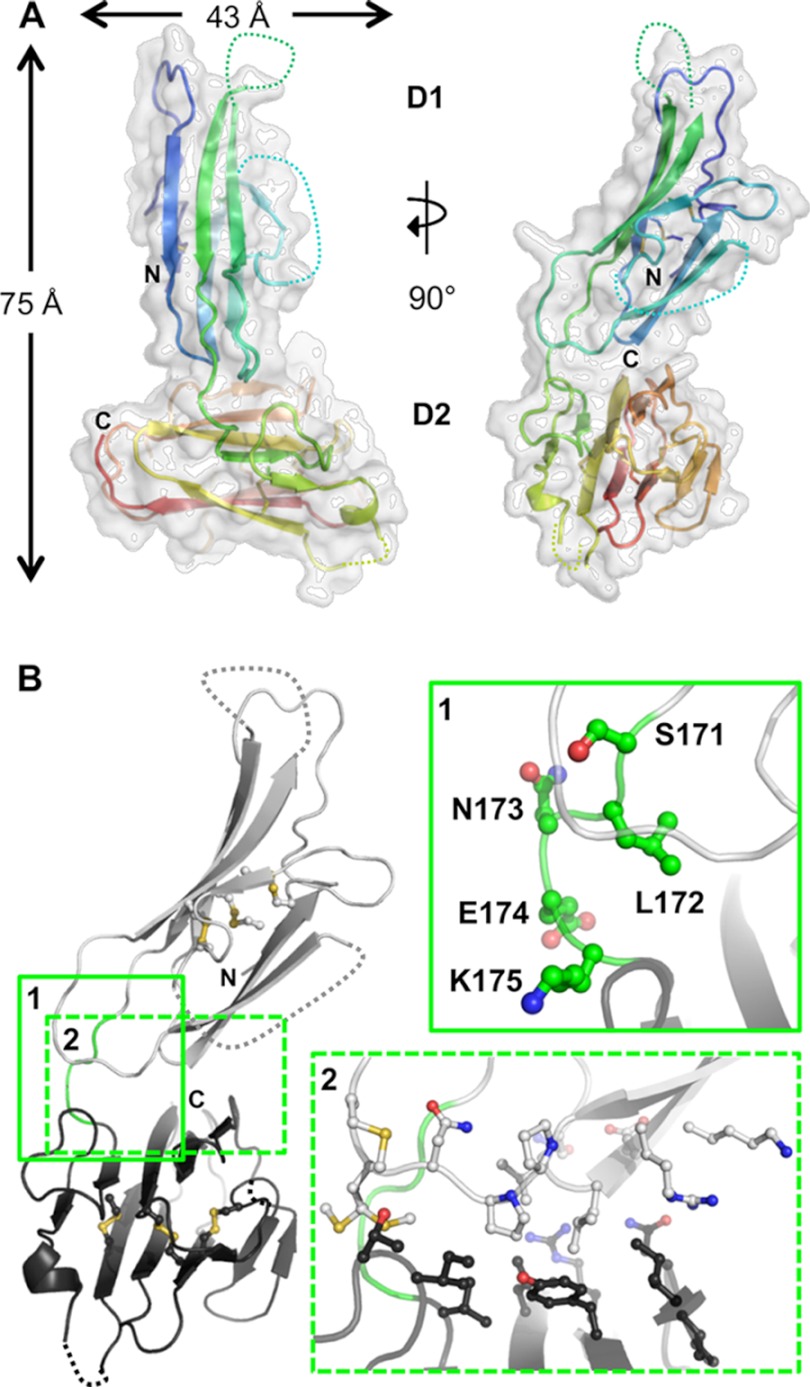
FIGURE 3.
Overall structure and interdomain organization of Pf12short. A, shown are orthogonal views of the structure of Pf12short. The semi-transparent white surface reveals an overall size of ∼75 Å tall by 43 Å wide. The secondary structure is shown beneath the surface as a schematic colored in a rainbow from the N terminus (blue) to the C terminus (red), with unmodeled regions indicated by dotted connecting loops. B, inset 1, analysis of the Pf12 inter-domain linker (Ser-171, Leu-172, Asn-13, Glu-174, and Lys-175) shows that no structurally constrictive or extremely hydrophobic residues are present. D1 is shown as light gray; D2 is in dark gray; linker is in green with side chains as balls and sticks. Inset 2, interfacing residues (as defined by PISA software), shown as balls and stick, reveal the lack of a significant interface between D1 and D2. All molecular figures were generated in PyMOL.