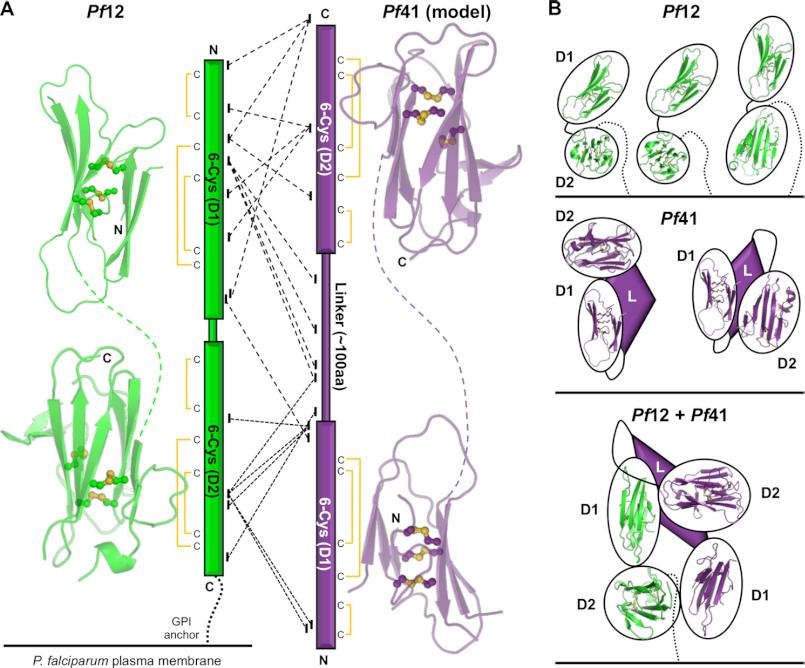
FIGURE 6.
Mass spectrometry and cross-linking confirm the Pf12-Pf41 heterodimeric interaction and suggest an antiparallel organization. A, left, shown is the Pf12 structure split into D1 and D2, highlighting the β-sandwich core (green schematic) and three disulfides (yellow balls and sticks) in each domain. Linker is shown as a green dashed line. Center, shown is a graphical representation of MS/MS results from analysis of the cross-linked Pf12long(NXA)-Pf41(NXA) heterodimer. Cross-links supporting the potential for an antiparallel organization, enabled by the lack of a Pf41 GPI anchor, are shown as dashed (emanating from Pf12 D1) and dotted (emanating from Pf12 D2) lines. Disulfide connectivity is indicated. Right, Pf41 D1 and D2 models generated off Pf12short D1 and D2 templates, respectively, highlighting the β-sandwich core (purple schematic) and three disulfides (yellow balls and sticks) in each domain. The linker is shown as a purple dashed line. B, schematics of the most likely organizations of Pf12 (top, green), Pf41 (middle, purple), and the Pf12-Pf41 complex are based on structural and cross-linking data. GPI anchors are shown as dotted lines. Pf41 linker with unknown structural elements are shown as purple diamonds. Black ovals around structurally characterized domains (Pf12 D1 and D2) and homology modeled domains (Pf41 D1 and D2) are presented for clarity of the general organization due to the uncertainty in the exact interacting molecular surfaces.