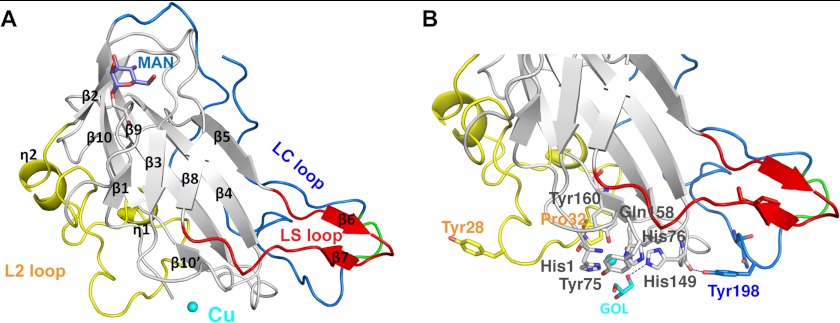
FIGURE 1.
Features of the PchGH61D crystal structure. A, schematic representation of the PchGH61D structure with the bound copper atom, depicted as a sphere in cyan. The L2 loop (residues 17–57) is colored in yellow, the short LS loop containing a β-hairpin in red (residues 109–124), and the C-terminal LC loop in blue (residues 170–217). All secondary structure elements of the enzyme are labeled according to their position in the protein sequence. The glycosylated residue Ser-11 and the attached O-linked mannose residue are shown in a stick representation in gray and slate blue, respectively. B, close up view of the structure showing potentially important residues at the proposed substrate-binding surface and the metal binding site in a stick representation with the same color coding as in A. The flexible portion of the LC loop (residues 201–204) is colored in green in both panels.