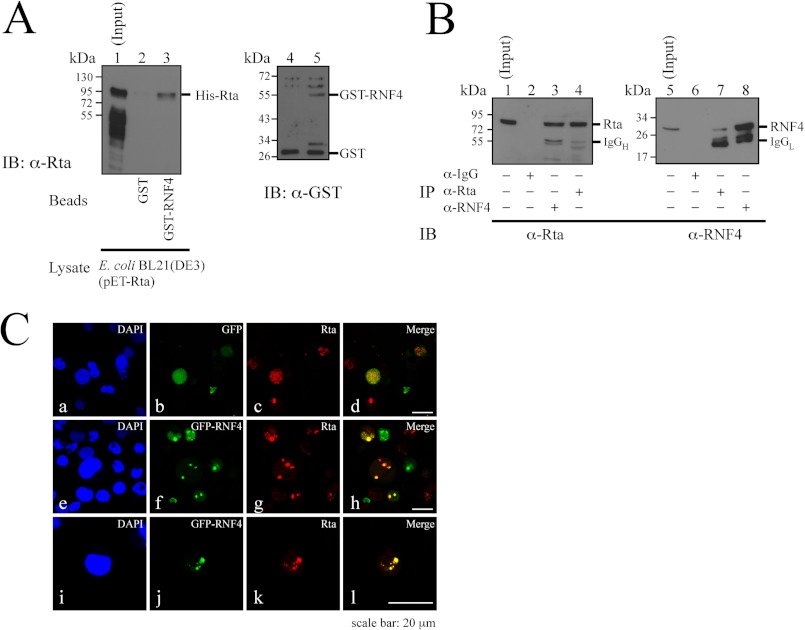
FIGURE 4.
Interaction between Rta and RNF4. A, bacterially expressed GST and GST-RNF4 were bound to glutathione-Sepharose beads. The beads were then mixed with a lysate from E. coli BL21(DE3)(pET-Rta). Proteins bound to GST-glutathione-Sepharose (lane 2) and GST-RNF4-glutathione-Sepharose (lane 3) beads were analyzed by immunoblotting (IB) using anti-Rta antibody. Lane 1 was loaded with 1% of the cell lysate. GST proteins that were bound to the beads were eluted and analyzed by immunoblot analysis using anti-GST antibody (lanes 4 and 5). B, P3HR1 cells were treated with TPA and sodium butyrate. Proteins in the lysate were subsequently immunoprecipitated (IP) using anti-IgG (lanes 2 and 6), anti-Rta (lanes 4 and 7), or anti-RNF4 (lanes 3 and 8) antibodies. Immunoblotting was performed using anti-Rta (lanes 1–4) and anti-RNF4 (lanes 5–8) antibodies. Input lanes were loaded with 1% of the lysates. IgGH, heavy chain of IgG; IgGL, light chain of IgG. C, P3HR1 cells were transfected with pEGFP-C1 (a–d) or pEGFP-RNF4 (e–l) and then treated with 5 μm MG132, sodium butyrate, and TPA for 24 h. Cells were incubated with anti-Rta monoclonal antibody. DAPI staining revealed the nucleus. Cells were observed under a confocal laser-scanning microscope. d, h, and l are merged images.