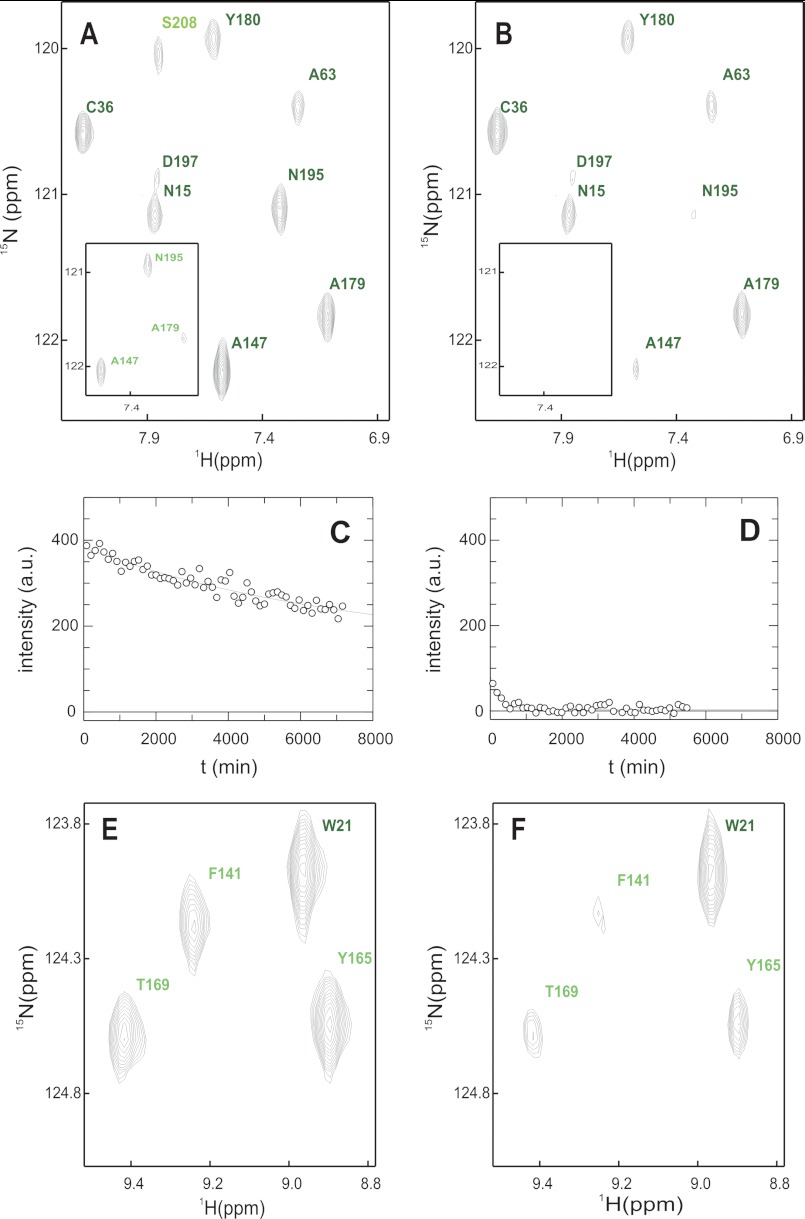
FIGURE 3.
Native state amide exchange of G3P*. A and B, section of the 1H15N-TROSY-HSQC in D2O buffer immediately after start (A) and after 600 min (B). The inset shows the same experiment in the presence of TolA-C. C and D, amide exchange kinetics of Ala-179 in the absence (C) and in the presence of TolA-C (D) including the single exponential fit. E and F, competition between refolding and amide exchange. E, section of the 1H15N-TROSY-HSQC in D2O buffer of native G3P. F, same section as in E but recorded after G3P was refolded in D2O buffer and the residual denaturant was removed by rapid gel filtration. Amide cross-signals are labeled and color-coded as in Fig. 8. B, D, and F, residues with protection factors larger than 10,000 are shown in dark green, values between 10,000 and 100 are in green, and values smaller than 100 are in pale green. The buffer was 50 mm sodium phosphate, pH 7.0 (or pD 6.6) in all cases.