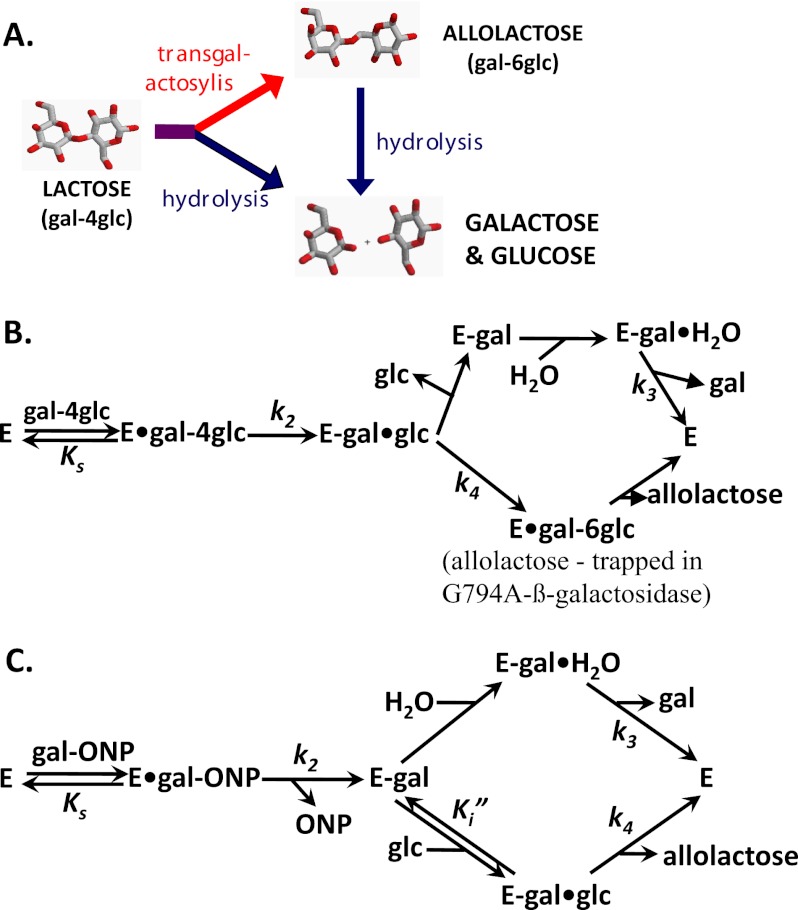
FIGURE 1.
The reactions of β-galactosidase. A, shown is the general scheme presenting the structures of lactose and allolactose. The concurrent hydrolysis and intramolecular transgalactosylis reactions are diagrammed. Allolactose production is only transitory because it is also hydrolyzed to Gal and Glc by β-galactosidase. B, shown is the reaction mechanism when lactose is the substrate. C, shown is the reaction mechanism when oNPG and Glc are substrates. The addition of glucose is used to study the transgalactosylation reaction. For B and C, Ks is the dissociation constant of the enzyme·substrate complex, k2 is the rate constant of the first displacement reaction, and k3 is the rate constant for the second displacement reaction when water reacts. For the transgalactosylation reaction with Glc that forms allolactose, the rate constant is k4. Ki″ is the dissociation constant for Glc dissociating from the covalent complex between the enzyme and Gal (E-Gal·Glc).