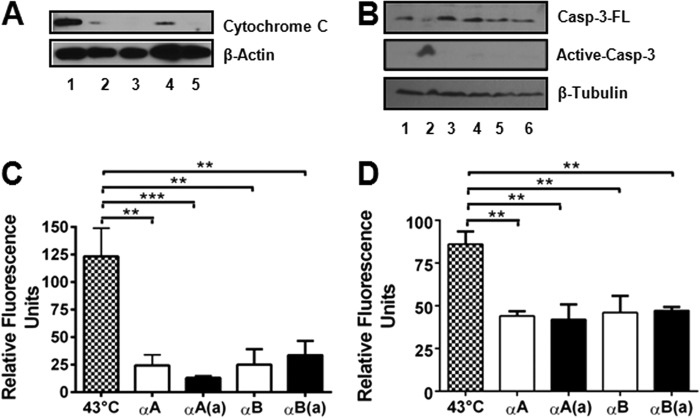
FIGURE 3.
The inhibition of hyperthermia-induced apoptosis by crystallin peptides occurs through blockade of the mitochondrial death pathway. CHO cells were treated with peptides and thermally stressed, as described in the legend to Fig. 2. α-Crystallin peptides inhibit cytochrome c release from the mitochondria (A). Cytochrome c release from the mitochondria into the cytosol was assessed via Western blotting. All lanes correspond to thermally stressed cells. Lane 1, no peptide; lane 2, +αA-native peptide; lane 3, +αA-acetyl peptide; lane 4, +αB peptide; and lane 5, +αB-acetyl peptide. Activation of procaspase-3 was inhibited by both acetyl and native peptides (B). Casp-3-FL, full-length procaspase-3; Active-casp-3, active caspase-3. Lane 1, control; lanes 2-6, thermally stressed cells; lane 2, no peptide; lane 3, +αA-native peptide; lane 4, +αA-acetyl peptide; lane 5, +αB peptide; and lane 6, +αB-acetyl peptide. Caspase-3 (C) and caspase-9 (D) activity was measured using specific fluorogenic substrates. Both αA-crystallin and αB-crystallin peptides inhibited caspase activity. The bars represent the mean ± S.D. of three independent experiments. αA, αA-native peptide; αA(a), αA-acetyl peptide; αB, αB-native peptide; and αB(a), αB-acetyl peptide. The differences between the native and acetyl peptides were not statistically significant. **, p < 0.005; ***, p < 0.0005.