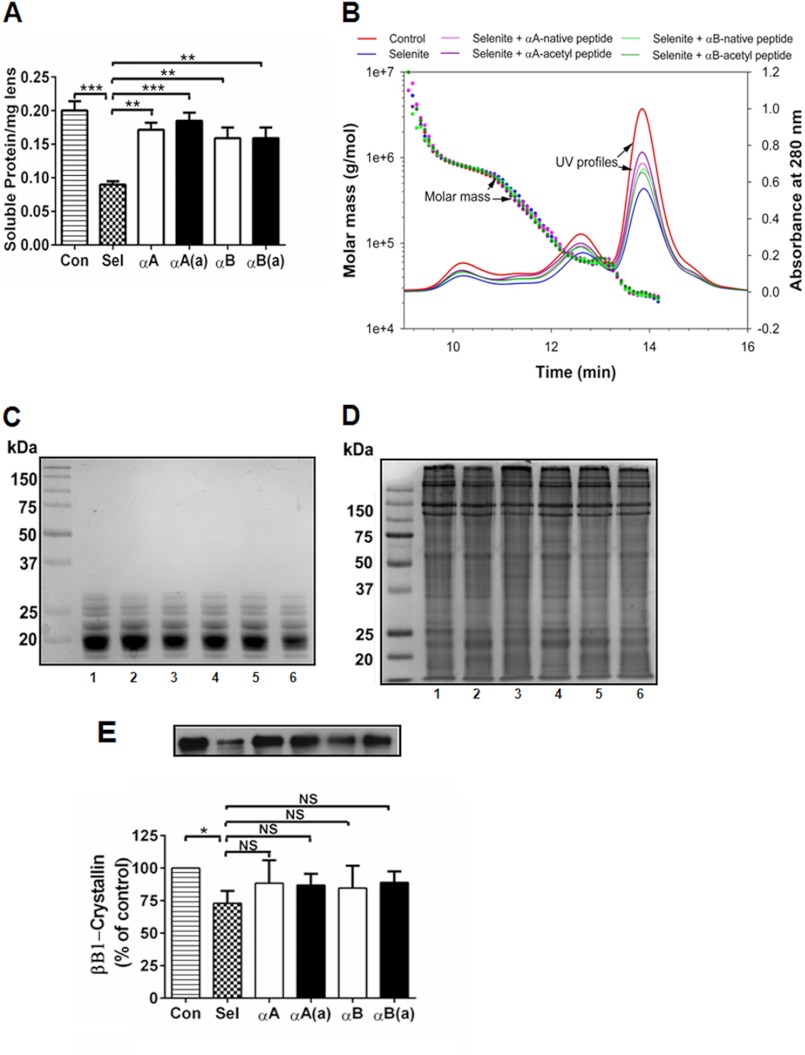
FIGURE 7.
In rats, α-crystallin peptides inhibit protein insolubilization in selenite cataracts. Cataracts were induced using sodium selenite, and the animals were treated with multiple 10-μg peptide injections, as described in the legend to Fig. 5. The lenses were harvested from animals on day 6-post sodium selenite injection and processed as described under “Experimental Procedures.” The water-soluble protein content decreased in the selenite-induced cataracts, which was significantly corrected by the native and acetyl peptides (panel A). MALS-DLS analyses of the water-soluble protein fraction from control, selenite-treated, and selenite + peptide-treated animals showed no apparent differences between groups (panel B). Water-soluble and insoluble proteins were analyzed by SDS-PAGE, which showed no additional protein cross-linking under either selenite or selenite + peptide treatment (panels C and D). 1, control; lanes 2–6, sodium selenite treated; 2, no peptide; 3, +αA-native peptide; 4, +αA-acetyl peptide; 5, +αB peptide; and 6, +αB-acetyl peptide. The cleavage of βB1-crystallin in the water-insoluble selenite cataract fraction (determined by Western blotting) was inhibited by peptide administration (panel E). The bars represent the mean ± S.D. of three independent experiments. The differences between the native and acetyl peptides were not statistically significant. *, p < 0.05; **, p < 0.005; ***, p < 0.0005. NS, not significant.