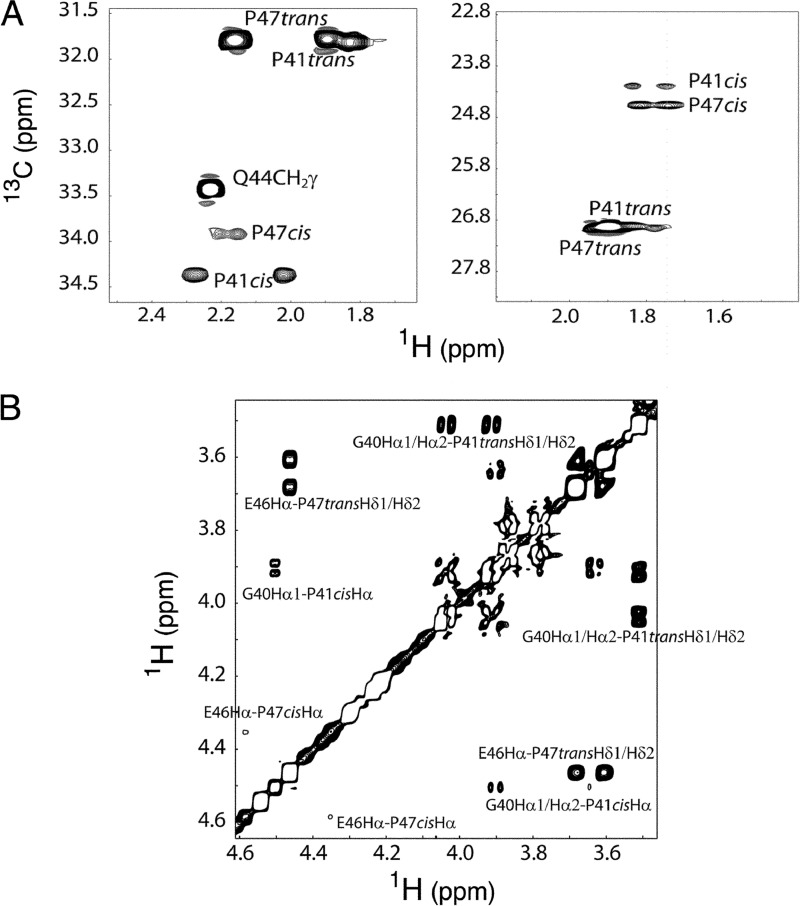
FIGURE 6.
E7Ep peptide proline chemical shift assignment. A, selected region of the 1H-13C HMQC spectrum showing the chemical shift assignment of CH2 groups of positions β (left) and γ (right) of prolines at 20 °C. For both Pro-41 (P41) and Pro-47 (P47), the trans isomer is much more abundant at equilibrium (85 and 95%, respectively). B, selected region of the NOESY spectrum at 20 °C showing the cis and trans chemical shift assignments. Characteristic Hδ-Hαi − 1 NOEs are observed for the trans prolines, whereas Hα-Hαi − 1 cross-peaks are detected in the cis isomers.