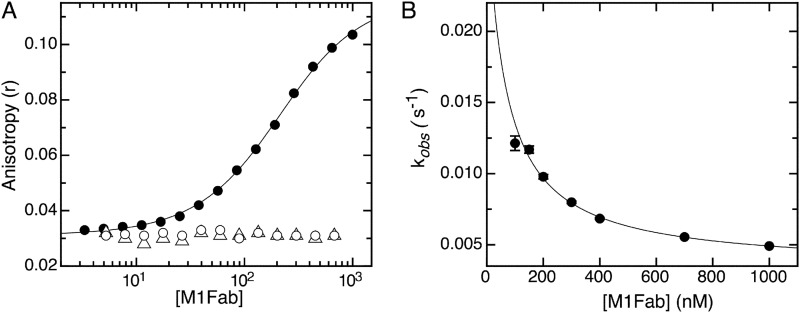
FIGURE 8.
Equilibrium binding and association kinetics of the complex of E7Ep proline mutants with M1Fab. A, FITC fluorescence anisotropy change measured upon adding increasing amounts of M1Fab to a cuvette containing 100 nm FITC- P47A E7Ep (filled circles), 100 nm FITC- P41A E7Ep (open circles), or 100 nm FITC- P41A + P47A E7Ep (open triangles). The different M1Fab·antigen complexes were incubated in separate tubes for 30 min prior to measurements. The line is a fit of the data for the FITC-P47A E7Ep·M1Fab complex to a 1:1 binding model, from which the following dissociation constant was obtained (see “Experimental Procedures”): KD = 160 ± 8 nm. The P41A E7Ep and the P41A/P47A E7Ep mutants did not bind to the M1Fab at the concentrations tested, which prevented fitting of the data. B, concentration dependence of the slow association phase for the FITC-P47A E7Ep·M1Fab complex measured at 50 nm P47A E7Ep and at increasing M1Fab concentrations. The observed rate constants were obtained from monoexponential fitting of the kinetic traces (supplemental Fig. S6), and the line represents the fitting of the data to a pre-equilibrium model by using Equation 4.