FIGURE 4.
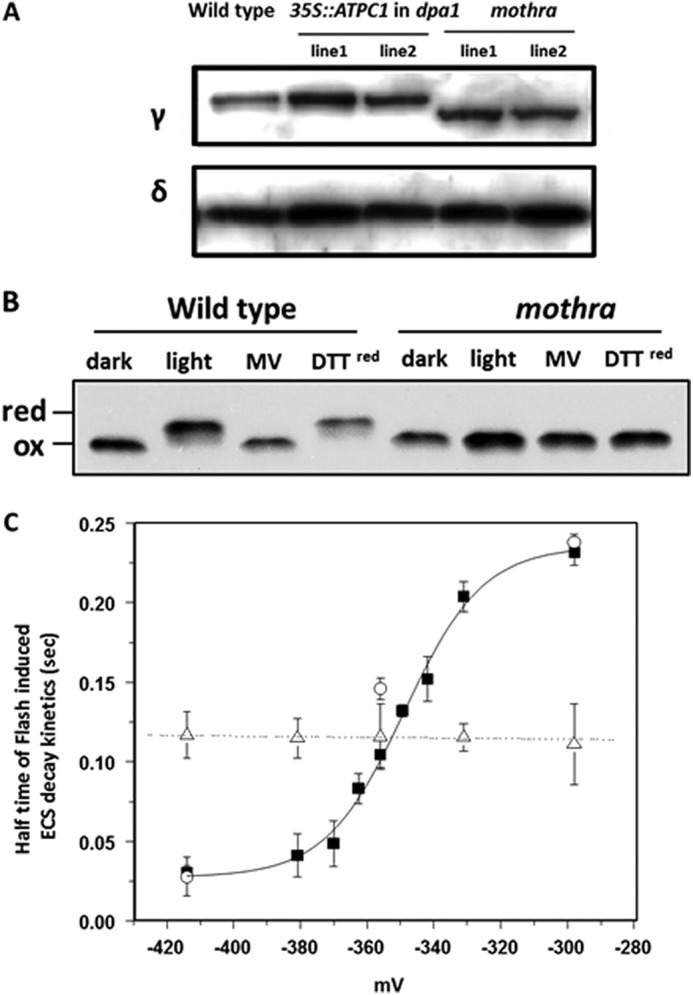
Accumulation and redox properties of chloroplast ATP synthase in wild type and mothra. A, accumulation of ATP synthase subunit γ and δ using Western blot analysis of thylakoid membrane proteins from wild type, representative complemented line (35S:: ATPC1 expressed in dpa1), and dpa1 complemented with modified ATPC1 (mothra). B, separation of reduced and oxidized γ subunits using AMS gel shift assay. To obtain reducing conditions (red), leaf discs were infiltrated with reagents and illuminated with ∼50 μmol photons m−2 s−1. Wild-type and mothra were treated with buffer as a control, methyl viologen (MV), and reduced DTT under light or dark condition. C, equilibrium redox titrations (18) of thiol/disulfide regulatory groups in the γ subunit of the chloroplast ATP synthase in wild type (filled squares), complemented line (35S:: ATPC1 expressed in dpa1) (open circles), and mothra (open triangles). The data for the wild-type titration were well fit with an n = 2 Nernst curve as expected for a disulfide/sulfhydryl transition. The ΔA520 relaxation kinetics were measured and used to calculate the halftime of the kinetics. Data represent the average ± S.D. (error bars) of n = 4–5.
