FIGURE 5.
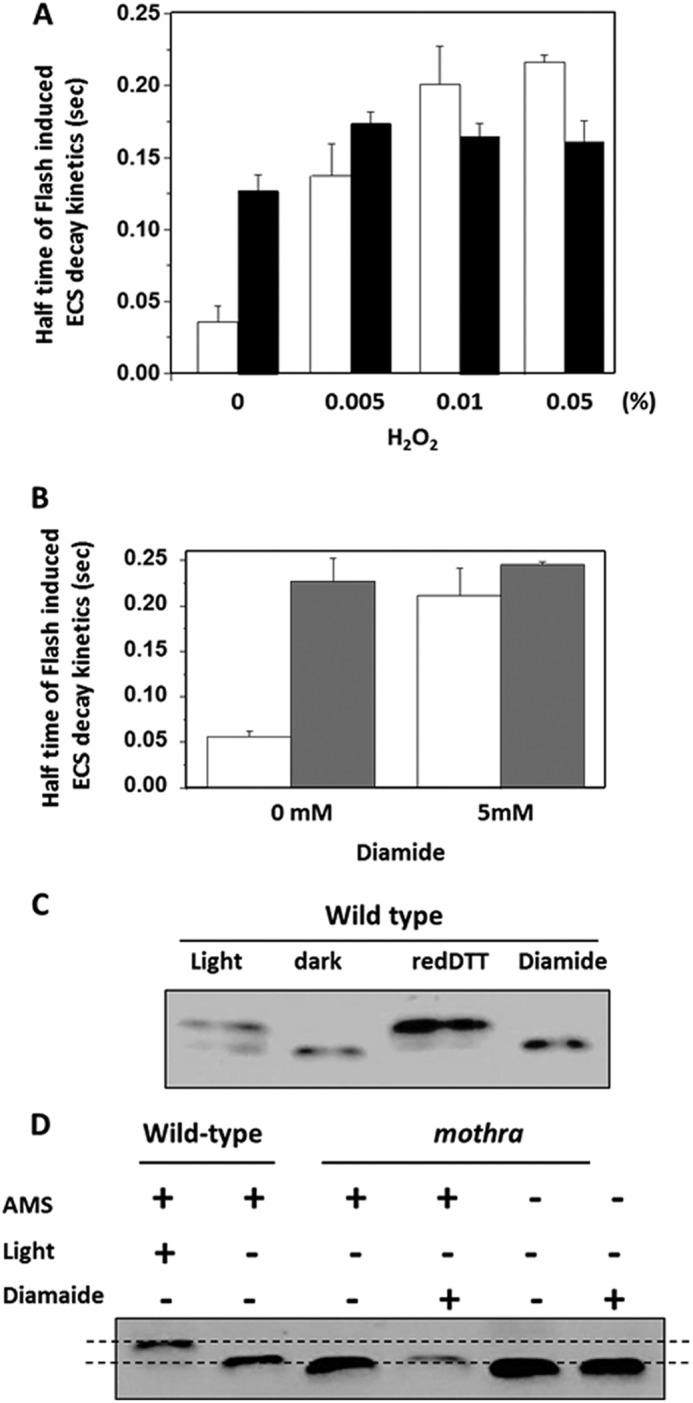
Modulation of ATP synthase activities and γ subunit structural properties by infiltration with strong oxidants. A, decay half-times of flash-induced ECS kinetics after preillumination, performed as described under “Experimental Procedures,” in leaf discs of wild-type (white bars) and mothra (black bars) infiltrated with buffer (20 mm Tricine-KOH, pH 7.5, 1% Tween 20) or a range of hydrogen peroxide (H2O2) concentrations. B, leaf discs of wild type (white bars) and mothra (gray bars) infiltrated as in A, but with 5 mm strong oxidant diamide. C, control demonstrating the use of AMS gel shift assay for assessing modulation of γ subunit redox state in wild type under conditions described in Ref. 17. As indicated in the figure, wild-type leaf discs were vacuum-infiltrated with buffer as in A, during illumination with 100 μmol photons m−2 s−1 or in darkness, reduced DTT, 5 mm diamide. Insoluble proteins were solubilized in sample buffer without addition of reducing regents, incubated in the thiol binding probe AMS, and separated by SDS-PAGE. Reduced proteins bands migrate less and appear in the upper part of the gel, whereas oxidized proteins migrate further (16, 17). D, AMS gel shift assay for redox state of wild-type and mothra γ subunit by light and diamide using the methods described for C. As a control, wild-type and leaf discs were treated with buffer in light (100 μmol photons m−2 s−1) or darkness, as described in Ref. 17. Leaf discs from mothra were treated with buffer and 5 mm diamide in the presence and absence of AMS labeling.
