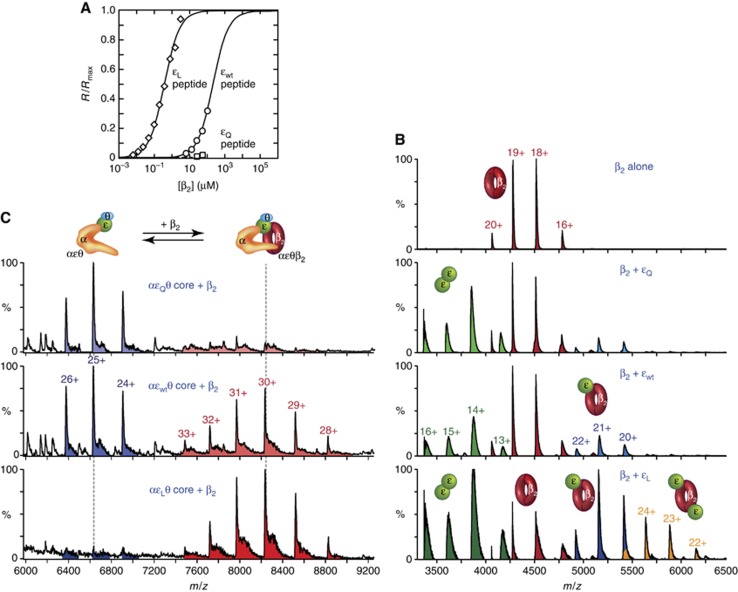
Figure 3.
Physical interaction occurs between β2 and the clamp-binding motif (CBM) of ε in the αεθ–β2 complex. (A) β2 binds to a peptide containing the εwt CBM. SPR binding isotherms (R/Rmax) for the interaction of β2 with immobilized decapeptides containing CBMs from εL (diamonds), εwt (circles), and εQ (squares) are shown; sensorgrams are in Supplementary Figure S3A. Fits to data using a 1:1 binding model (εL and εwt peptides) are shown as solid lines. The small responses with the εQ peptide at the highest [β2] indicate KD>2 mM (see Supplementary Figure S3A). (B) The CBM in εL is accessible to β2. NanoESI mass spectra of 1 μM β2 alone or with 20 μM εwt, εQ, or εL show that εL interacts more strongly with β2 than does εwt. Proteins were in 140 mM NH4OAc, and ions due to free monomeric ε (1700–3400 m/z) have been omitted for clarity. (C) Wild-type ε contacts β2 in the αεθ–β2 complex, shown by a shift in the αεθ: αεθβ2 equilibrium (in excess β2) with progressive increase in ε–β binding strength. NanoESI-MS of 2.8 μM β2 with 1.8 μM purified αεθ cores in 140 mM NH4OAc. Ions due to free β2 (4000–5000 m/z) are not shown.