Abstract
Microfabricated fluidic systems have emerged as a powerful approach for chemical analysis. Relatively unexplored is the use of microfabrication to create sampling probes. We have developed a sampling probe microfabricated in Si by bulk micromachining and lithography. The probe is 70 μm wide by 85 μm thick by 11 mm long and incorporates two buried channels that are 20 μm diameter. The tip of the probe has two 20 μm holes where fluid is ejected or collected for sampling. Utility of the probe was demonstrated by sampling from the brain of live rats. For sampling, artificial cerebral spinal fluid was infused in through one channel at 50 nL/min while sample was withdrawn at the same flow rate from the other channel. Analysis of resulting fractions collected every 20 min from the striatum of rats by liquid chromatography with mass spectrometry demonstrated reliable detection of 17 neurotransmitters and metabolites. The small probe dimensions suggest it is less perturbing to tissue and can be used to sample smaller brain nuclei than larger sampling devices, such as microdialysis probes. This sampling probe may have other applications such as sampling from cells in culture. The use of microfabrication may also enable incorporation of electrodes for electrochemical or electrophysiological recording and other channels that enable more complex sample preparation on the device.
Introduction
Microfabrication techniques have fostered a revolution in chemical measurement by allowing multiple analytical operations such as mixing, separation, and detection to be integrated in microfluidic circuits.1–4 These devices offer improved automation, performance, portability, and efficiency of sample and reagent usage. Much less attention has been paid to using microfabrication to create microfluidic sampling devices. Microfabrication may allow development of sampling probes that are miniaturized for sampling from microenvironments, incorporate sample processing steps, and create samples that better match the low volume requirements of microfluidic devices. In this work we describe a sampling probe fabricated in Si using lithographic patterning and bulk micromachining. The probe is demonstrated for sampling from the brain of live animals.
In vivo chemical measurement is an important approach in neuroscience because it allows neurotransmitter dynamics to be measured in intact systems and correlated to behavior. Microdialysis is well-established for in vivo sampling5–11; however, microdialysis probes are typically 200 to 500 μm diameter with 1 to 4 mm long sampling area, resulting in relatively poor spatial resolution. Miniaturization of sampling probes is highly desirable because small clusters of cells control specific functions in the brain.12 Tissue damage due to probe insertion13, which has the potential to confound measurements, may also be reduced by probe miniaturization.
Spatial resolution can be improved by using low-flow push-pull perfusion in which sample is withdrawn through one capillary and make-up fluid is pumped from another, closely spaced, capillary.14 In this way, sampling occurs just at the probe tip.14, 15 Tissue perturbation is kept low by using flow rates < 50 nL/min. These probes have been constructed from fused silica capillary tubes such that the total diameter is 220 to 440 μm.14, 16, 17 Although low-flow push-pull perfusion has improved spatial resolution, the limitations of fused silica capillary led us to employ microfabrication which could offer smaller probe dimensions and potential for incorporating more functions into the sampling system.
Microneedles with small overall dimensions have been microfabricated in metal18, 19, polymer20, 21, glass22, 23, Si24–26, and ceramic27. These devices provide precedent for creating small fluidic elements; but, they have only been used for chemical delivery (e.g, microinjection) rather than sampling. A “chemistrode” microfabricated in PDMS has been reported for sampling by segmented flow; however, the overall dimensions are not suitable for insertion into tissue and the sampling method requires flow against a surface.28 Microfabrication of a microdialysis probe has been reported, but not tested for sampling.29 We report microfabrication of low-flow push-pull sampling probes with small overall dimensions. The probes were used in striatum of live rat brain to demonstrate feasibility of sample collection from a complex environment. Samples were analyzed with liquid chromatography–electrospray ionization-mass spectrometry (LC-ESI-MS) to measure 17 neurochemicals.
Experimental
Design and microfabrication of low flow push-pull probe
Sampling probes were designed in L-EDIT software (Tanner EDA). The final product was 11 mm in length, 84 μm in width along the shank, and 70 μm thick with 2 fluidic channels of 20 μm inner diameter (ID) buried in the probes (see Figure 1). Orifices for inlet and outlet were placed 10 μm above the probe sampling tip. Ports were created on the opposite end for connection to pumps and sample collection devices. Probes were fabricated on 4 inch silicon-on-insulator (SOI) wafers (University Wafer, MA) which had a 70 μm thick Si layer over a 1 μm thick insulator layer over a 500 μm thick Si wafer. The fabrication process, which is based on buried channel technology (BCT)30, is outlined in Figure 2 with details given in Supporting Information.
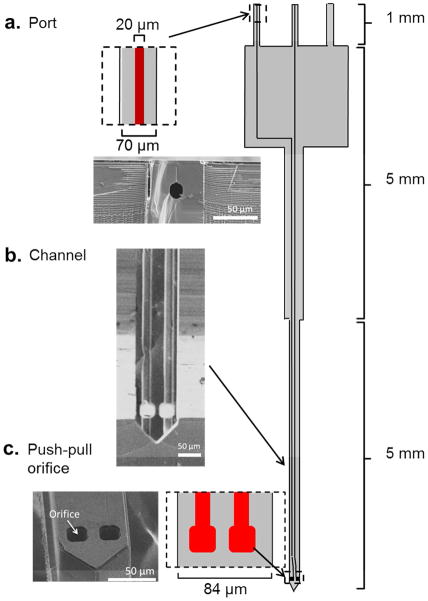
Figure 1.
Layout of probe with scanning electron micrographs of different sections. Drawing at right shows the probe layout. Dark lines in interior are buried channels with 20 μm diameter. (a) Port where connection is made to capillaries. Drawing shows close view and micrograph shows cross section from a probe that was broken to show the buried channel. (Probes had 3 ports. Two were in use for these designs; the third is available for a possible 3rd fluidic arm, but was not used in this work). (b) Micrograph of channel after backside etching to reveal the channels. Slight defect is due to partial unfinished backside etching. (c) Close up drawing and micrograph of the sampling orifice.
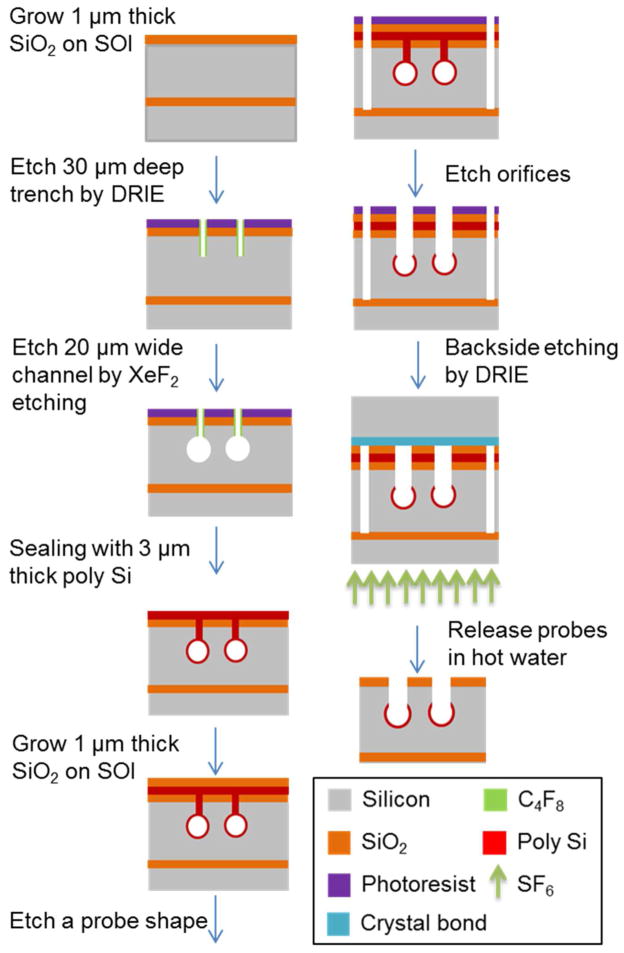
Figure 2.
Sequential steps for microfabrication of Si probe. Device is shown in cross-section. 20 μm wide channels were formed using Xactix XeF2 (isotropic etching) through 30 μm deep trenches and sealed with poly silicon. Deep reactive ion etching (DRIE) then cut probe shapes and sampling orifices. Probes were thinned by the backside etching by DRIE on a silicon support and released in hot water. See Experimental in supporting information for details.
In Vivo Sampling
Si sampling probes were inserted steretoxically over a period of 2 min into the striatum through a burr hole in the skull to a depth of 5 mm (see supporting information for surgical and sampling details).31 Probes were perfused at 50 nL/min (each channel). After 1 h of perfusion, a total of 4 to 8 fractions (corresponding to 80 to 160 min sampling time) of 1 μL each were collected from each of three animals. Samples were derivatized with benzoyl chloride and analyzed by LC-ESI-MS, as described elsewhere.32 Analytes were quantified as the ratio of analyte to 13C-labeled internal standards in comparison to a standard curve.
Results and Discussion
Probe fabrication
Probes were fabricated into the design shown in Figure 1. The length of probes is sufficient to reach deep brain structures in laboratory animals. The tip and shank dimensions (70 × 84 μm) present a surface area (~6,000 μm2) to tissue that is just 15% of that in previous push-pull designs or small microdialysis probes (~36,000 μm2) so that much less tissue is displaced during sampling. Si was used as the material because it could provide the rigidity necessary to be inserted into tissue for such thin probesand is easily processed. Approximately 150 probes are fabricated per processing run on a single 4 inch wafer allowing high efficiency of production.
A challenge in fabricating microneedle devices is enclosing a fluidic channel given the intrinsic stresses and fragility of such structures. The approach we used is based on buried channel technology (BCT).30, 33 In this process, lithographically defined trenches are dug by deep reactive ion etching. Trenches are expanded by isotropic etching then sealed (see Figure 2 and supporting information.) This process yielded straight probes and round, smooth channels with good fidelity (Figure 1). Wet anisotropic etching has previously been utilized for fabrication of microneedles, but presents difficulty in fabrication of tees, corners, and other fluidic geometries due to rapid etching of convex edges.34 Other techniques for fabricating channels were considered such as the assembly of two fabricated substrate materials with bonding35, surface micromachining with 3D BCT,36 and use of sacrificial material.29 Compared to these methods, we found BCT effective because it has no alignment error as possible during bonding two substrates, higher smoothness and mechanical strength of a channel wall than 3D BCT, and low cost compared with sacrificial material method due to short term of the furnace process.
Probe holder
Another challenge in utilizing microfluidic devices for sampling is the need to couple devices to the “outside world” with leak-free connections for delivery of fluid and removal of analytes from a sampling probe. For removing sample at low flow rates, the dead volume of the connection must be low. For connections, a port was fabricated on top of the probe that was 70 μm square and 1 mm long. To connect these ports to fused silica capillaries, we microfabricated a probe holder that allowed the port channel and lumen of a connector capillary to be aligned and sealed inside a “union” fused silica capillary (see Supporting Information Figure S1 and Experimental for details). While some dead volume is inevitable, the probe holder allowed convenient coupling between probe and capillaries and provided a “handle” for manipulating the probe.
In vivo sampling
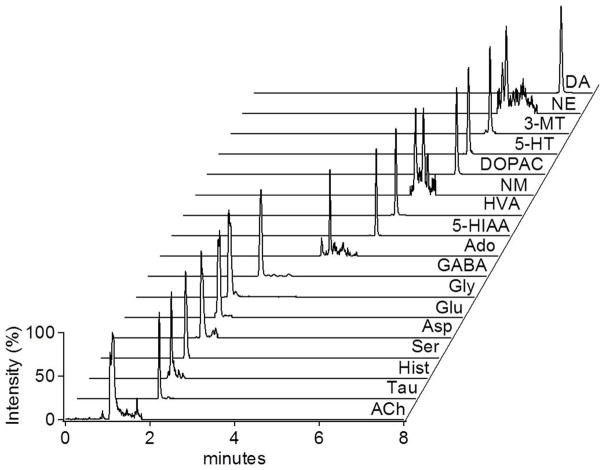
To demonstrate utility of the Si probes, we used them to sample from the striatum of anesthetized rats. Figure 3 illustrates a chromatogram for 17 neurotransmitters and metabolites from a single, 1 μL fraction collected at 50 nL/min. The average concentration of analytes detected by this method are (in nM ± SEM): acetylcholine = 26 ± 12, adenosine = 39 ± 21, aspartate = 530 ± 110, dopamine = 19 ± 7, γ-aminobutyric acid = 78 ± 30, glutamate = 1100 ± 500, glycine = 3000 ± 600, histamine = 5.9 ± 1.5, norepinephrine = 1.5 ± 1.3, and serotonin = 0.36 ± 0.16. In vivo concentrations were not corrected for recovery, but we found recovery to be 93 ± 5% (n = 3) for fluorescein in a stirred vial. It is difficult to compare results between studies because many variables that can affect recovered concentrations such as probe recovery, anesthesia, and animal age. Nevertheless it is apparent that pattern of concentrations is mostly as expected (see Table S1 for comparison data).37–45 Acetylcholine and dopamine are typically low nanomolar while GABA and adenosine are somewhat higher. Norepinepherine is expected to be substantially lower than dopamine in striatum. Glutamate is generally in the low micromolar range. Several reports of aspartate show low nanomolar concentration but we observed 530 nM. This may relate to the anesthesia. Variation was higher than reported for microdialysis which may be due to greater variation when sampling from a smaller space, low number of replicates, or experimental artifacts (noise in low volume measurements or measurements taken soon after probe implant). Further work is required to better understand these effects. Sampling was completed for up to 160 min (longer times were not attempted) with no failures or clogging showing the potential for reliable operation in tissue. These results show that it is possible to reliably collect quantifiable amounts of all the low molecular weight neurotransmitters and many of their metabolites by this approach.
Figure 3.
Mass chromatograms of a representative fraction (1 μL) collected from the striatum of an anesthetized through the Si probe. The trace for each of the 17 neurotransmitters and metabolites labeled were from a particular MS → MS transition monitored by LC-MS as described elsewhere.32 Abbreviations used are: acetylcholine (ACh), taurine, histamine (Hist), serine (Ser), aspartate (Asp), glycine (Gly), glutamate (Glu), γ-aminobutyric acid (GABA), adenosine, 5-hydroxyindoleacetic acid (5-HIAA), homovanillic acid (HVA), normetanephrine, 3,4-dihydroxyphenylacetic acid (DOPAC), serotonin (5-HT), 3-methoxytyramine (3-MT), norepinephrine (NE), dopamine (DA).
We did not attempt to measure concentration changes in the brain; however, in vitro tests showed that sampling through the probe we could record concentration changes with 26 s response time (Figure S2). This time would be the best possible temporal resolution with the current configuration; however, practically temporal resolution will be limited by the amount of sample needed for analysis. Histological examination of the sampling sites where dye was infused showed remarkably localized lesions (Figure S3) relative to that typically seen for microdialysis or push-pull perfusion suggesting the potential for lower tissue damage.
Conclusions
Bulk micromachining of silicon wafers is a viable way to fabricate push-pull sampling probes. The small size of the probes relative to other in vivo sampling probes results in the ability to place the probe in smaller brain regions. Further, these probes displace less tissue than other sampling probes and therefore result in less tissue disruption. Although small samples are recovered, it is possible to use LC-MS to measure neurotransmitters and metabolites. Microscale analytical methods like CE or capillary LC may be better suited for analyzing the small fractions17, 46 and will allow better temporal resolution. The probes have intriguing potential for further development and other applications. It may be possible fabricate electrodes onto the probes for simultaneous electrical or electrochemical measurements.47 It may also be possible to fabricate additional channels for sample preparation (e.g., derivatization or to segment flow for improved temporal resolution). While the probes were applied to brain tissue, they may also have application to other microenvironments such cultured cells. It may be of interest to use the probe for selective delivery of reagents or drugs using hydrodynamic confinement principles reported for microfabricated pipettes.20
Supplementary Material
Acknowledgments
This work was supported by the McKnight Foundation and NIH R37 EB003320.
References
- 1.Harrison DJ, Fluri K, Seiler K, Fan Z, Effenhauser CS, Manz A. Science. 1993;261:895–897. doi: 10.1126/science.261.5123.895. [DOI] [PubMed] [Google Scholar]
- 2.Verpoorte EMJ, Schoot BHVD, Jeanneret S, Manz A, Widmer HM, Rooij NFD. J Micromech Microeng. 1994;4:246. [Google Scholar]
- 3.Broyles BS, Jacobson SC, Ramsey JM. Anal Chem. 2003;75:2761–2767. doi: 10.1021/ac025503x. [DOI] [PubMed] [Google Scholar]
- 4.Whitesides GM. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 5.Davies MI, Cooper JD, Desmond SS, Lunte CE, Lunte SM. Adv Drug Del Rev. 2000;45:169–188. doi: 10.1016/s0169-409x(00)00114-9. [DOI] [PubMed] [Google Scholar]
- 6.Kehr J. J Neurosci Methods. 1993;48:251–261. doi: 10.1016/0165-0270(93)90096-a. [DOI] [PubMed] [Google Scholar]
- 7.Kehr J, Yoshitake T, Wang FH, Wynick D, Holmberg K, Lendahl U, Bartfai T, Yamaguchi M, Hokfelt T, Ogren SO. J Neurosci Methods. 2001;109:71–80. doi: 10.1016/s0165-0270(01)00403-4. [DOI] [PubMed] [Google Scholar]
- 8.Ao X, Stenken JA. Methods. 2006;38:331–341. doi: 10.1016/j.ymeth.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Herrera-Marschitz M, Goiny M, You ZB, Meana JJ, Pettersson E, Rodriguez-Puertas R, Xu ZQ, Terenius L, Hokfelt T, Ungerstedt U. Neurosci Biobehav Rev. 1997;21:489–495. doi: 10.1016/s0149-7634(96)00033-4. [DOI] [PubMed] [Google Scholar]
- 10.Watson CJ, Venton BJ, Kennedy RT. Anal Chem. 2006;78:1391–1399. doi: 10.1021/ac0693722. [DOI] [PubMed] [Google Scholar]
- 11.Westerink BH, Cremers TI. Handbook of microdialysis: methods, applications and perspectives. Academic Press; Amsterdam, Netherlands: 2007. [Google Scholar]
- 12.Pecina S, Berridge KC. J Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL. J Neurosci Methods. 1999;90:129–142. doi: 10.1016/s0165-0270(99)00064-3. [DOI] [PubMed] [Google Scholar]
- 14.Kottegoda S, Shaik I, Shippy SA. J Neurosci Methods. 2002;121:93–101. doi: 10.1016/s0165-0270(02)00245-5. [DOI] [PubMed] [Google Scholar]
- 15.Slaney TR, Mabrouk OS, Porter-Stransky KA, Aragona BJ, Kennedy RT. ACS Chem Neurosci. 2012 doi: 10.1021/cn300158p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slaney TR, Nie J, Hershey ND, Thwar PK, Linderman J, Burns MA, Kennedy RT. Anal Chem. 2011;83:5207–5213. doi: 10.1021/ac2003938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cellar NA, Burns ST, Meiners JC, Chen H, Kennedy RT. Anal Chem. 2005;77:7067–7073. doi: 10.1021/ac0510033. [DOI] [PubMed] [Google Scholar]
- 18.Chandrasekaran S, Brazzle JD, Frazier AB. J Microelectromech Syst. 2003;12:281–288. [Google Scholar]
- 19.Davis SP, Martanto W, Allen MG, Prausnitz MR. IEEE Trans Biomed Eng. 2005;52:909–915. doi: 10.1109/TBME.2005.845240. [DOI] [PubMed] [Google Scholar]
- 20.Ainla A, Jeffries GD, Brune R, Orwar O, Jesorka A. Lab Chip. 2012;12:1255–1261. doi: 10.1039/c2lc20906c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon SJ, Lee SS, Lee H, Kwon T. Microsyst Technol. 2005;11:311–318. [Google Scholar]
- 22.Adamo A, Jensen KF. Lab Chip. 2008;8:1258–1261. doi: 10.1039/b803212b. [DOI] [PubMed] [Google Scholar]
- 23.Wang PM, Cornwell M, Hill J, Prausnitz MR. J Invest Dermatol. 2006;126:1080–1087. doi: 10.1038/sj.jid.5700150. [DOI] [PubMed] [Google Scholar]
- 24.Lin L, Pisano AP. J Microelectromech Syst. 1999;8:78–84. [Google Scholar]
- 25.Gardeniers HJGE, Luttge R, Berenschot EJW, De Boer MJ, Yeshurun SY, Hefetz M, van’t Oever R, van den Berg A. J Microelectromech Syst. 2003;12:855–862. [Google Scholar]
- 26.Chen J, Wise KD, Hetke JF, Bledsoe SC., Jr IEEE Trans Biomed Eng. 1997;44:760–769. doi: 10.1109/10.605435. [DOI] [PubMed] [Google Scholar]
- 27.Verhoeven M, Bystrova S, Winnubst L, Qureshi H, de Gruijl TD, Scheper RJ, Luttge R. Microelectron Eng. 2012 [Google Scholar]
- 28.Chen D, Du W, Liu Y, Liu W, Kuznetsov A, Mendez FE, Philipson LH, Ismagilov RF. Proc Natl Acad Sci U S A. 2008;105:16843–16848. doi: 10.1073/pnas.0807916105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zahn JD, Trebotich D, Liepmann D. IEEE. 2000. pp. 375–380. [Google Scholar]
- 30.de Boer MJ, Tjerkstra RW, Berenschot J, Jansen HV, Burger G, Gardeniers J, Elwenspoek M, van den Berg A. J Microelectromech Syst. 2000;9:94–103. [Google Scholar]
- 31.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Elsevier; Amsterdam, Netherlands: 2007. [Google Scholar]
- 32.Song P, Mabrouk OS, Hershey ND, Kennedy RT. Anal Chem. 2012;84:412–419. doi: 10.1021/ac202794q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fekete Z, Pongracz A, Furjes P, Battistig G. Microsyst Technol. 2012;18:353–58. [Google Scholar]
- 34.Pal P, Sato K, Chandra S. J Micromech Microeng. 2007;17:R111. [Google Scholar]
- 35.Grétillat MA, Paoletti F, Thiébaud P, Roth S, Koudelka-Hep M, De Rooij N. Sens Actuators, A. 1997;60:219–222. [Google Scholar]
- 36.Zellner P, Renaghan L, Hasnain Z, Agah M. J Micromech Microeng. 2010;20:045013. [Google Scholar]
- 37.Gerhardt GA, Maloney RE., Jr Brain Res. 1999;816:68–77. doi: 10.1016/s0006-8993(98)01095-6. [DOI] [PubMed] [Google Scholar]
- 38.Wang M, Roman GT, Perry ML, Kennedy RT. Anal Chem. 2009;81:9072–9078. doi: 10.1021/ac901731v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morari M, O’Connor WT, Ungerstedt U, Fuxe K. J Neurochem. 1993;60:1884–1893. doi: 10.1111/j.1471-4159.1993.tb13416.x. [DOI] [PubMed] [Google Scholar]
- 40.Lillrank SM, O’Connor WT, Oja SS, Ungerstedt U. J Neural Transm Gen Sect. 1994;95:145–155. doi: 10.1007/BF01276433. [DOI] [PubMed] [Google Scholar]
- 41.Bert L, Parrot S, Robert F, Desvignes C, Denoroy L, Suaud-Chagny MF, Renaud B. Neuropharmacology. 2002;43:825–835. doi: 10.1016/s0028-3908(02)00170-3. [DOI] [PubMed] [Google Scholar]
- 42.Hows ME, Organ AJ, Murray S, Dawson LA, Foxton R, Heidbreder C, Hughes ZA, Lacroix L, Shah AJ. J Neurosci Methods. 2002;121:33–39. doi: 10.1016/s0165-0270(02)00228-5. [DOI] [PubMed] [Google Scholar]
- 43.Pinna A, Corsi C, Carta AR, Valentini V, Pedata F, Morelli M. Eur J Pharmacol. 2002;446:75–82. doi: 10.1016/s0014-2999(02)01818-6. [DOI] [PubMed] [Google Scholar]
- 44.Herrera-Marschitz M, You ZB, Goiny M, Meana JJ, Silveira R, Godukhin OV, Chen Y, Espinoza S, Pettersson E, Loidl CF, Lubec G, Andersson K, Nylander I, Terenius L, Ungerstedt U. J Neurochem. 1996;66:1726–1735. doi: 10.1046/j.1471-4159.1996.66041726.x. [DOI] [PubMed] [Google Scholar]
- 45.Tossman U, Jonsson G, Ungerstedt U. Acta Physiol Scand. 1986;127:533–545. doi: 10.1111/j.1748-1716.1986.tb07938.x. [DOI] [PubMed] [Google Scholar]
- 46.Hogan BL, Lunte SM, Stobaugh JF, Lunte CE. Anal Chem. 1994;66:596–602. doi: 10.1021/ac00077a004. [DOI] [PubMed] [Google Scholar]
- 47.Seidl K, Spieth S, Herwik S, Steigert J, Zengerle R, Paul O, Ruther P. J Micromech Microeng. 2010;20:105006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.