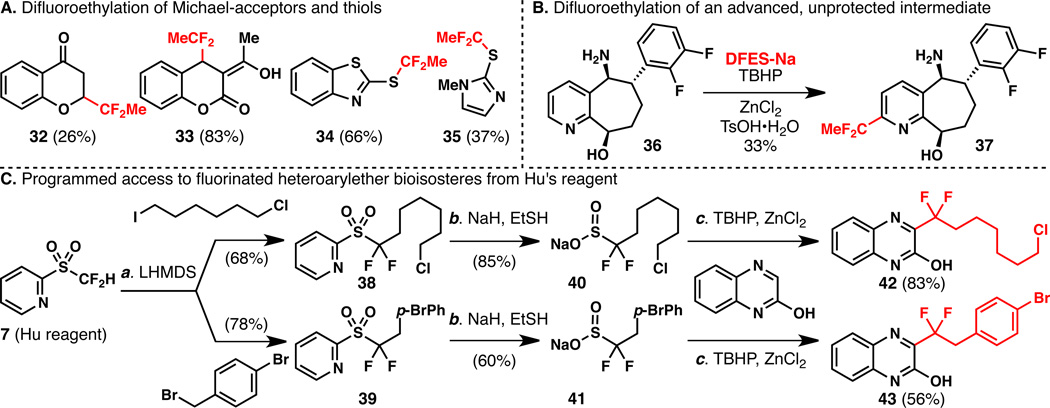
Figure 2.
A. Direct difluoroethylation of heteroaromatic Michael acceptors and thiols. B. Direct difluoroethylation of an advanced intermediate from the Bristol-Myers Squibb compound library. C. A general synthesis of fluorinated heteroarylether bioisosteric reagents and a proof of concept on 2-quinoxalinol. Reagents and conditions: (a) reactions performed on 0.2 mmol scale, 40 (2.0 equiv), TBHP (5.0 equiv), TsOH•H2O (1.0 equiv), ZnCl2 (1.0 equiv), 0 to 23 °C, reaction completed in 24 h; (b) reactions performed on 0.1 mmol scale, 41 (1.5 equiv), TBHP (5.0 equiv), TsOH•H2O (1.0 equiv), ZnCl2 (1.5 equiv), reaction completed in 4 h at 0 °C after second addition of 41 (1.5 equiv), TBHP (5.0 equiv).