Abstract
In China, esophageal cancer is the fourth leading cause of cancer death where essentially all cases are histologically esophageal squamous cell carcinoma (ESCC), in contrast to esophageal adenocarcinoma in the West. Globally, ESCC is 2.4 times more common among men than women and recently it has been suggested that sex hormones may be associated with the risk of ESCC. We examined the association between genetic variants in sex hormone metabolic genes and ESCC risk in a population from north central China with high-incidence rates. A total of 1026 ESCC cases and 1452 controls were genotyped for 797 unique tag single-nucleotide polymorphisms (SNPs) in 51 sex hormone metabolic genes. SNP-, gene- and pathway-based associations with ESCC risk were evaluated using unconditional logistic regression adjusted for age, sex and geographical location and the adaptive rank truncated product (ARTP) method. Statistical significance was determined through use of permutation for pathway- and gene-based associations. No associations were observed for the overall sex hormone metabolic pathway (P = 0.14) or subpathways (androgen synthesis: P = 0.30, estrogen synthesis: P = 0.15 and estrogen removal: P = 0.19) with risk of ESCC. However, six individual genes (including SULT2B1, CYP1B1, CYP3A7, CYP3A5, SHBG and CYP11A1) were significantly associated with ESCC risk (P < 0.05). Our examination of genetic variation in the sex hormone metabolic pathway is consistent with a potential association with risk of ESCC. These positive findings warrant further evaluation in relation to ESCC risk and replication in other populations.
Introduction
Esophageal cancer causes more than 400 000 deaths each year and is the sixth leading cause of cancer death worldwide (1,2). Morbidity and mortality rates for esophageal cancer in China are high in northern areas, including Shanxi and Henan Provinces where essentially all cases of esophageal cancer are esophageal squamous cell carcinoma (ESCC) as opposed to adenocarcinomas, which predominate in the Western world (3). Although smoking tobacco and drinking alcoholic beverages account for nearly 90% of ESCC cases in Western countries including the USA (4–6), these exposures explain very little of the risk in high-risk populations in China (7–10). Low levels of dietary vitamins and minerals, consumption of pickled food and exposure to nitrosamines (3,7,8) have been investigated but not convincingly linked to the high incidence, suggesting that these environmental risk factors alone may not be responsible for ESCC. Several lines of evidence for a genetic influence in these high incidence regions exist, including studies on family history, segregation analysis and association studies (9–17).
Globally, esophageal cancer is two to four times more common among men than women (2). For ESCC specifically, and similar to other upper aerodigestive tract cancers, the male:female incidence ratio is 2.4:1 (2). Both epidemiological and experimental evidence suggest that sex hormones may play an important role in the development of ESCC. Epidemiological studies have reported that users of hormone therapy have a lower risk of ESCC compared with never users (18–20), and an inverse association has also been observed among oral contraceptive users (20). Wang et al. (21) recently compared serum estradiol levels in age-matched, healthy subjects from high- and low-incidence areas of ESCC in Henan, China. Significantly reduced estradiol was observed in patients with ESCC from the high-incidence area compared with healthy controls from both high- and low-incidence areas suggesting a role for low estrogen with increased risk of ESCC (21). Normal esophageal epithelia and ESCC also express both estrogen receptors ESR1 and ESR2 (22–26), and the androgen receptor (AR) (27,28). The limited data available suggest that estrogen per se may be protective against ESCC (26). Thus, genes involved in sex hormone metabolism may be important in the etiology of ESCC and genetic variation in such genes may affect risk of developing ESCC.
We comprehensively evaluated candidate genes in the sex hormone metabolic pathway using a tag single-nucleotide polymorphism (SNP) approach and present data here suggesting that specific genes involved in this pathway may be important for ESCC development in high-risk Chinese individuals.
Materials and methods
Study population
Data and DNA samples for this study were derived from two upper gastrointestinal (UGI) cancer projects: the case–control component of the UGI Cancer Genetics Project (Shanxi) and a nested case–cohort study within the Linxian Nutrition Intervention Trial (NIT).
The Shanxi case–control study was conducted between 1997 and 2000 and included newly diagnosed, histologically confirmed ESCC cases with no prior history of treatment (10). Cases from five geographic regions (Taiyuan, Linfen, Jinzhong, Chanzi and Xinzhou) were identified and enrolled following admission to the Shanxi Cancer Hospital in Taiyuan. Age- (+/– 5 years) and neighborhood-matched controls were identified and enrolled within 6 months of each ESCC case. A further 500 controls from the gastric cancer arm of the Shanxi study and from the same five geographic regions were also included for analyses. Risk factor information obtained by interview and a blood sample for DNA analysis were obtained at enrollment for both cases and controls.
The NIT trial (29,30) was initiated in Linxian in 1985 as a randomized trial that tested four vitamin–mineral combinations taken daily for 5.25 years on the incidence and mortality of esophageal cancer. In 1999 and 2000, ~16 000 living NIT participants gave a blood sample for genetic analyses. Since the conclusion of the trial in 1991, follow-up of all participants for vital status and cancer endpoints has continued. Based on follow-up through 2006, ESCC cases diagnosed since blood draw along with age- and sex-stratified randomly sampled subcohort were selected for evaluation in this study. In addition, controls were randomly selected without regard to the study arm. Risk factor information for the NIT cohort was obtained at the start of the intervention in 1985.
Gene and SNP selection
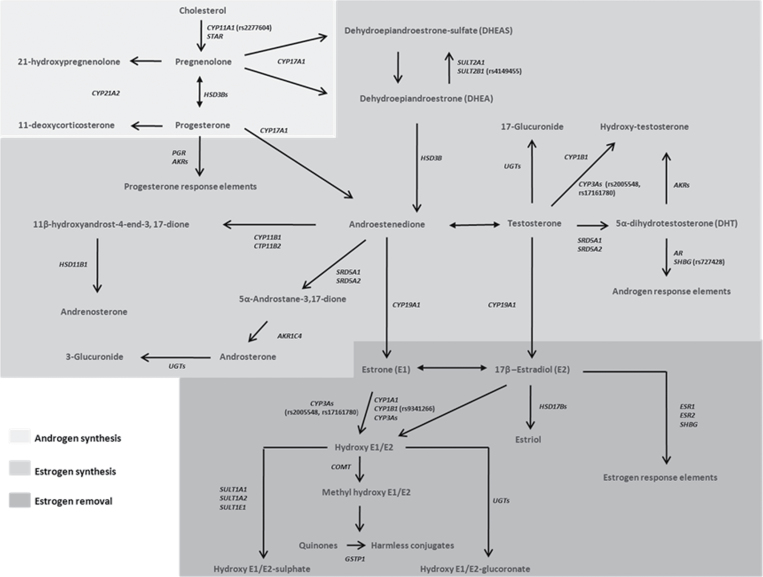
The ‘Rare Cancers iSelect Project’ was organized in the Summer/Fall of 2007 and solicited candidate genes and molecular pathway ideas from investigators in the Division of Cancer Epidemiology and Genetics at the NCI. Overall, 20 000 subjects from 15 different cancer studies were genotyped on the same high-dimension custom SNP array (iSelect Illumina) that contained over 29 000 SNPs and included tags for 1316 genes. Tag SNPs were selected across European (CEU), Japanese (YRI) and Chinese (CHB) populations from Hapmap and linkage disequilibrium (LD) information in the regions of interest in the International HapMap Project (Release 24 with National Center for Biotechnology Information build 36, www.hapmap.org/). Candidate sex hormone metabolizing and sex hormone-related genes such as transporters and signaling receptors (collectively referred to here as sex hormone metabolic genes) were identified from the literature (31–34) and cross referenced with the Kyto Encyclopedia of Genes and Genomes (KEGG) pathway database (35) to confirm pathway information. We identified 54 autosomal sex hormone metabolic genes containing 1109 unique tag SNPs from the iSelect project. Selected tag SNPs were located within 20kb upstream or 10kb downstream of a gene; had a minor allele frequency ≥5% in the HapMap cohort of European (CEU), Chinese (CHB) and Japanese (YRI) populations; and were not already represented by a current tag SNP at an linkage equilibrium of r 2 ≥ 0.80. Because SNP selection was based on two ethnic groups in HapMap, some of the SNPs in our study population have a minor allele frequency of <5%. Figure 1 illustrates the 54 genes that are involved in the overall sex hormone metabolic pathway as well as the three subpathways examined (androgen synthesis, estrogen synthesis and estrogen removal).
Fig. 1.
Illustration showing the overall sex hormone metabolic pathway and subpathways. Genes (in italics) involved in androgen synthesis (light gray background), estrogen synthesis (or androgen to estrogen conversion, medium gray background) and estrogen removal (dark gray background) are indicated (Figure adapted from Li et al. (33) and Yang et al. (34)). Androgen synthesis genes: cytochrome P450 (CYP), family 11, subfamily A, polypeptide 1 (CYP11A), CYP17A1, hydroxysteroid (3-beta) dehydrogenase 1 (HSD3B1), HSD3B2, CYP21A2 and steroidogenic acute regulatory protein (STAR). Estrogen synthesis gene: Aldo-keto reductase family 1 member C1 (AKR1C1), AKR1C2, AKR1C3, AKR1C4, CYP11B1, androgen receptor (AR), CYP11B2, CYP19A1, hydroxysteroid (11-beta) dehydrogenase 1 (HSD11B1), HSD3B1, HSD3B2, progesterone receptor (PGR), steroid 5-alpha-reductase (SRD5A1), SRD5A2, DHEA sulfotransferase (SULT2A1), hydroxysteroid sulfotransferase (SULT2B1), UDP-glucuronosyltransferase 1 family (UGTA1), UGT1A10, UGT1A3, UGT1A4, UGT1A5, UGT1A6, UGT1A7, UGT1A8, UGT1A9, UGT2A1, UGT2A3, UGT2B4, UGT2B7, UGT2B10, UGT2B11, UGT2B17, UGT2B28, CYP1B1, CYP3A4, CYP3A5, CYP3A7 and SHBG. Estrogen removal genes: CYP1B1, catechol-O-methyltransferase (COMT), CYP1A1, CYP3A4, CYP3A5, CYP3A7, estrogen receptor 1 (ESR1), ESR2, glutathione S-transferase p1 (GSTP1), HSD17B1, HSD17B2, HSD17B3, HSD17B4, SHBG, SULT1A1, SULT1A2, estrogen sulfotransferase (SULT1E1), UGT1A1, UGT1A10, UGT1A3, UGT1A4, UGT1A5, UGT1A6, UGT1A7, UGT1A8, UGT1A9, UDP-glucuronosyltransferase 2 family A1 (UGT2A1), UGT2A3, UGT2B10, UGT2B11, UGT2B4 and UGT2B7. Steroid sulfatase isozyme S (STS), N-acetyltransferase 1 (NAT1), NAT2 and NAD(P)H dehydrogenase, quinone 1 (NQO1) genes are not shown because these genes were not included in our analyses. The most significant SNP from each of the six significant genes associated with ESCC risk in this study is also highlighted in parentheses beside the gene. For additional information on assignment of the remaining tag SNPs to their relevant gene and subpathway, see Supplementary Table 6, available at Carcinogenesis Online.
Genotyping, quality control and exclusions
DNAs from a combined total of 1142 cases and 1700 controls were genotyped at the Core Genotyping Facility of the National Cancer Institute’s Division of Cancer Epidemiology and Genetics using a custom iSelect bead chip (Illumina Custom Infinium, http://www.illumina.com/pages.ilmn?ID=158). Further details and description of the method can be found at http://cgf.nci.nih.gov/operations/multiplex-genotyping.html.
We excluded SNPs with <95% completion and <95% concordance, a minor allele frequency (MAF) <1%, and when two of three genotype categories for either cases or controls had <5 subjects. Androgen receptor (AR), UGT2B17 and UGT2B28 genes contained only tag SNPs with MAFs <1%; therefore, these genes (and their 23 SNPs) were omitted from further analyses. Cases and controls were included in our analysis if >85% of SNPs were successfully genotyped in that person. After application of the exclusion criteria, 797 unique tag SNPs in 51 sex hormone metabolic genes remained. After excluding subjects with low completion rate (<85%), the final analysis for each tumor outcome included 1026 ESCC cases and 1452 controls. We further computed LD between any two SNPs in the same gene among controls using Haploview (http://www.broad.mit.edu/mpg/haploview/).
Statistical analyses
To investigate variation in sex hormone metabolic pathway genes and risk of ESCC in our study population, we carried out individual SNP-, gene-, and pathway-based analyses. SNP-based analyses were tested under the additive model and odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using unconditional logistic regression with adjustment for age (5 year categories), sex and geographical location in primary models. For a small number of SNPs, we modeled certain alleles as dominant because of the low frequency of the homozygous genotype in our population. In secondary models, we also adjusted for alcohol, smoking and family history of UGI cancer. In addition, we conducted analyses stratified by sex, smoking status and alcohol consumption, and examined potential effect modification between strata using a likelihood ratio test. All P values for SNPs are nominal except where otherwise specified. After excluding SNPs with pairwise LD r 2 ≥ 0.80 in controls, a Bonferroni-corrected threshold was calculated from the 494 SNPs (P = 1.01E-04, 0.05/494 SNPs). SNP-based analyses were performed using STATA version 9.0 and R language and multiple comparisons were adjusted by permutation testing using the program language R (http://www.r-project.org/).
For the gene-based analysis, the minP statistic, which was the minimum P value among all P values from the single SNP analysis conducted on SNPs within the candidate gene, was used to evaluate the association between a candidate gene and risk of ESCC as described previously (36). Analyses were implemented in the R package ARTP (http://dceg.cancer.gov/bb/tools/artp), and we used 20 000 bootstrap iterations in ARTP in each gene-based test.
The overall pathway- and subpathway-based P values were calculated based on genes (34,36). The P values across all relevant genes in the subpathway and/or overall pathway were combined using the ARTP method and permutated as described previously (36). The overall pathway P value was based on 51 genes, which contained a total of 1250 SNPs (collective figure accounts for one or more unique SNPs occurring in multiple genes). Subpathway P values were based on the combined test statistics of: 6 genes (total of 60 SNPs) for androgen synthesis, 35 genes (total of 923 SNPs) for estrogen synthesis and 32 genes (total of 920 SNPs) for estrogen removal. Statistical significance for all analyses was defined as P < 0.05.
Results
Population characteristics
A total of 1026 ESCC cases and 1452 controls from the combined studies were analyzed in this study. Demographic and risk factor information for each individual study and the combined population is shown in Table I. Cases were more likely to be male than female and have a family history of UGI cancer compared with controls. In the individual studies, the mean age for cases was lower in Shanxi (57.4±8.2) compared with NIT (64.0±7.6). Although the proportion of males was equivalent in cases and controls from NIT, the proportion of males in Shanxi (72%) was greater due to inclusion of additional controls from the gastric cancer arm of the UGI Cancer Genetics. Also, as a result of the recruitment of more females than males (55:45%) into the original prospective NIT cohort, the proportion of male cases in the NIT was lower than in Shanxi (Table I). Tobacco and alcohol use were both higher in Shanxi than in NIT, a reflection of the gender differences between studies because these exposures are uncommon in females.
Table I.
Population characteristics in combined and individual Chinese population studies
| Linxian Nutrition Intervention Trial | Shanxi UGI Cancer Genetics Project | Combined population | ||||
|---|---|---|---|---|---|---|
| Abbreviation | NIT | Shanxi | ||||
| Location | Henan Province, People’s Republic of China | Shanxi Province, People’s Republic of China | ||||
| Matching | Sex and age (10 year groups) | Sex and age (±5 years) | ||||
| Controls (n = 410) | Cases (n = 480) | Controls (n = 1042) | Cases (n = 546) | Controls (n = 1452) | Cases (n = 1026) | |
| Age (years) | 63.6±7.6 | 64.3±7.0 | 58.3±8.7 | 57.4.3±8.2 | 59.7±8.7 | 60.6±8.4 |
| %Male | 45% | 45% | 72% | 63% | 64% | 55% |
| % Smoking, yes | 31% | 28% | 64% | 58% | 55% | 44% |
| % Alcohol, yes | 25% | 23% | 50% | 47% | 43% | 36% |
| % Family History, UGI Cancer, yes | 32% | 37% | 20% | 25% | 24% | 31% |
SNP-based analyses
We identified 62 SNPs across 26 sex hormone metabolic genes (including CYP11A1, CYP11B1, CYP11B2, CYP17A1, CYP19A1, CYP1B1, CYP3A4, CYP3A5, CYP3A7, ESR1, ESR2, GSTP1, HSD17B2, SHBG, SRD5A2, SULT1E1, SULT2B1, UGT1A1, UGT1A3, UGT1A4, UGT1A5, UGT1A6, UGT1A, UGT1A8, UGT1A9 and UGT1A10) that were significantly associated (P < 0.05) with ESCC risk in the combined population (Supplementary Table 1, available at Carcinogenesis Online). Following LD computation using r 2 ≥ 0.80, these SNPs were shown to represent 51 independent signals across the 26 genes. SNP results in both independent studies (Shanxi and NIT) are shown in Supplementary Table 1, available at Carcinogenesis Online. The strongest association with ESCC risk in the combined study population was observed for rs4149455 in SULT2B1 (per allele OR: 0.81, 95% CI: 0.72–0.92, P = 0.0007) (Table II). Other significant SNP-based associations (P < 0.01) with risk of ESCC included rs9341266 CYP1B1 (per allele OR: 1.45, 95% CI: 1.16–1.82, P = 0.001); rs2277604 CYP11A1 (ORDominant: 1.45, 95% CI: 1.13–1.90, P = 0.004); rs12506209 SULT1E1 (per allele OR: 1.19, 95% CI: 1.06–1.34, P = 0.004); rs1529041 SULT1E1 [per allele OR: 0.78, 95% CI: 0.66–0.9, P = 0.004 (also in LD r 2 = 0.89 with rs1220832 SULT1E1)]; rs4281899 UGT1A8 (per allele OR: 1.17, 95% CI: 1.04–1.32, P = 0.007); rs17161780 CYP3A5 (per allele OR: 1.19, 95% CI: 1.04–1.35, P = 0.009); and rs6760588 within the UGT1A7-A10 gene cluster (per allele OR: 1.17, 95% CI: 1.04–1.31, P = 0.009) (Supplementary Table 1, available at Carcinogenesis Online). Further adjustment for smoking, alcohol and family history of UGI cancer did not alter these results (data not shown). No individual SNP remained significant after Bonferroni correction (P = 1.01E-04, 0.05/494 SNPs) for multiple comparisons.
Table II.
Pathway-, gene- and most significant SNP-based P values for sex hormone metabolic genes and risk of ESCC in the combined population
| Pathway P* | Gene | Chromosome | Number of SNPs | Gene P◊ | Most significant SNP | SNP P n |
|---|---|---|---|---|---|---|
| 0.14 | SULT2B1 | 19 | 22 | 0.013 | rs4149455 | 0.0007 |
| CYP1B1 | 2 | 30 | 0.019 | rs9341266 | 0.001 | |
| CYP3A7 | 7 | 2 | 0.030 | rs2005548 | 0.019 | |
| CYP3A5 | 7 | 8 | 0.034 | rs17161780 | 0.009 | |
| SHBG | 17 | 5 | 0.037 | rs727428 | 0.010 | |
| CYP11A1 | 15 | 15 | 0.038 | rs2277604 | 0.004 | |
| SULT1E1 | 4 | 16 | 0.052 | rs12506209 | 0.004 | |
| CYP3A4 | 7 | 5 | 0.062 | rs2005548 | 0.019 | |
| CYP11B2 | 8 | 7 | 0.097 | rs11781082 | 0.022 | |
| UGT1A1 | 2 | 26 | 0.102 | rs11888492 | 0.011 | |
| UGT1A3 | 2 | 41 | 0.118 | rs11888492 | 0.011 | |
| UGT1A4 | 2 | 46 | 0.128 | rs11888492 | 0.011 | |
| UGT1A5 | 2 | 54 | 0.155 | rs11888492 | 0.011 | |
| CYP11B1 | 8 | 5 | 0.157 | rs4736349 | 0.040 | |
| ESR2 | 14 | 30 | 0.169 | rs2987983 | 0.013 | |
| UGT1A7 | 2 | 68 | 0.171 | rs6760588 | 0.010 | |
| UGT1A6 | 2 | 64 | 0.175 | rs11888492 | 0.011 | |
| UGT1A8 | 2 | 84 | 0.176 | rs4281899 | 0.007 | |
| GSTP1 | 11 | 7 | 0.184 | rs625978 | 0.048 | |
| UGT1A9 | 2 | 73 | 0.189 | rs6760588 | 0.010 | |
| UGT1A10 | 2 | 76 | 0.208 | rs6760588 | 0.010 | |
| HSD17B2 | 16 | 57 | 0.253 | rs6564958 | 0.014 | |
| SRD5A2 | 2 | 21 | 0.262 | rs2300700 | 0.049 | |
| CYP21A2 | 6 | 1 | 0.274 | rs1742113 | 0.271 | |
| CYP17A1 | 10 | 16 | 0.328 | rs4919682 | 0.043 | |
| CYP19A1 | 15 | 70 | 0.434 | rs10519301 | 0.025 | |
| UGT2A3 | 4 | 6 | 0.454 | rs17147016 | 0.127 | |
| STAR | 13 | 7 | 0.467 | rs2070348 | 0.094 | |
| AKR1C3 | 10 | 23 | 0.476 | rs11597709 | 0.167 | |
| HSD17B4 | 5 | 24 | 0.499 | rs25640 | 0.106 | |
| ESR1 | 6 | 61 | 0.554 | rs3798577 | 0.021 | |
| SULT2A1 | 19 | 13 | 0.575 | rs2932760 | 0.122 | |
| HSD3B1 | 1 | 9 | 0.582 | rs3765944 | 0.237 | |
| HSD3B2 | 1 | 12 | 0.644 | rs11579951 | 0.167 | |
| UGT2B7 | 4 | 8 | 0.654 | rs7668282 | 0.214 | |
| AKR1C1 | 10 | 7 | 0.678 | rs7909151 | 0.225 | |
| AKR1C2 | 10 | 18 | 0.695 | rs12411321 | 0.112 | |
| SULT1A2 | 16 | 3 | 0.716 | rs4788073 | 0.347 | |
| COMT | 22 | 30 | 0.730 | rs1012157 | 0.089 | |
| CYP1A1 | 15 | 11 | 0.743 | rs11632814 | 0.188 | |
| UGT2A1 | 4 | 19 | 0.749 | rs10033854 | 0.132 | |
| AKR1C4 | 10 | 13 | 0.775 | rs4880711 | 0.190 | |
| UGT2B11 | 4 | 1 | 0.819 | rs12502502 | 0.814 | |
| HSD17B3 | 9 | 45 | 0.837 | rs8190480 | 0.086 | |
| SRD5A1 | 5 | 32 | 0.884 | rs8192166 | 0.179 | |
| SULT1A1 | 16 | 2 | 0.887 | rs1968752 | 0.670 | |
| UGT2B4 | 4 | 11 | 0.896 | rs17671289 | 0.265 | |
| PGR | 11 | 14 | 0.900 | rs471767 | 0.321 | |
| HSD17B1 | 17 | 5 | 0.946 | rs2071046 | 0.715 | |
| UGT2B10 | 4 | 5 | 0.953 | rs6816491 | 0.535 | |
| HSD11B1 | 1 | 22 | 0.974 | rs17317033 | 0.275 |
The overall sex hormone metabolic pathway P value (P*) was calculated by combining the P values of all 51 genes, using the ARTP method. Each gene-based P value (P◊) is the minimum P value among all P values from the single SNP analysis conducted on SNPs within each of the respective candidate genes. The genes are listed in order of lowest P◊, and those with significant P◊ values are bolded. SNP P values (P n) are the nominal P value of the most significant SNP in each gene. Statistical significance was determined by permutation testing (20 000).
Gene-based analyses
Gene-based analyses identified six genes significantly associated with ESCC risk (ARTP P < 0.05) (Table II and Supplementary Table 2, available at Carcinogenesis Online), including SULT2B1 (P = 0.013), CYP1B1 (P = 0.019), CYP3A7 (P = 0.030), CYP3A5 (P = 0.034), SHBG (P = 0.037) and CYP11A1 (P = 0.038). Results for the remaining 45 sex hormone metabolizing genes including their most significant SNP are shown in Table II. Further details for the six significant genes are shown in Table III.
Table III.
Genes significantly associated with risk of ESCC in the overall sex hormone pathway in the combined population
| Gene | Location | Gene.P◊ | Total number of tag SNPs | No. of SNPs with P < 0.05 (%) | Most significant SNP (major/minor allele) | Most significant SNP* | ||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P n | ||||||
| SULT2B1 | 19q13.3 | 0.013 | 22 | 2 (9%) | rs4149455 (C/T) | 0.81 | 0.72–0.92 | 0.0007 |
| CYP1B1 | 2p21 | 0.019 | 30 | 1 (3.3%) | rs9341266 (G/A) | 1.45 | 1.16–1.82 | 0.001 |
| CYP3A7 | 7q21–q22.1 | 0.030 | 2 | 1 (50%) | rs2005548 (C/T) | 1.17 | 1.03–1.32 | 0.019 |
| CYP3A5 | 7q21.1 | 0.034 | 8 | 4 (50%) | rs17161780 (G/A) | 1.19 | 1.04–1.35 | 0.009 |
| SHBG | 17p13-p12 | 0.037 | 5 | 3 (60%) | rs727428 (T/C) | 0.86 | 0.76–0.96 | 0.010 |
| CYP11A1 # | 15q23-q24 | 0.038 | 15 | 1 (7%) | rs2277604 (G/T) | 1.45 | 1.13–1.90 | 0.004 |
Gene-based P values (P◊) were calculated using the ARTP method. SNP* OR and nominal P values (P n) were obtained from unconditional logistic regression. Per allele ORs and nominal P values for SNPs were obtained from unconditional logistic regression and were adjusted by age, sex and geographical location. CYP11A1 rs2277604 was analyzed using a dominant model (#), whereas the remaining SNP ORs are per allele ORs.
Pathway-based analyses
Pathway-based analyses for all 51 genes in the overall sex hormone metabolic pathway did not show significant association with ESCC risk (P = 0.14) (Table II). In addition, the three subpathways evaluated [androgen synthesis (6 genes), estrogen synthesis (35 genes) and estrogen removal pathways (32 genes) (Figure 1)], also showed no significant associations with risk of ESCC (Supplementary Table 3, available at Carcinogenesis Online).
Sex stratification and gene-based pathway analysis
Because ESCC incidence is higher in men than women worldwide (2), we examined the association of the six significant genes (i.e. SULT2B1, CYP1B1, CYP3A7, CYP3A5, SHBG and CYP11A1) separately in males and females (Table IV). The most significant SNP in CYP11A1 (rs2277604) showed a markedly stronger effect in males (ORDominant: 1.90, CI: 1.34–2.69) (interaction P = 0.034) than in females (ORDominant: 1.10, CI: 0.73–1.53) (Table IV). In males, the overall sex hormone metabolic pathway was suggestive but not significant (P = 0.063) and the androgen subpathway was significantly associated with ESCC risk (P = 0.038). No pathway/subpathways showed significant associations in women (Supplementary Tables 4 and 5, available at Carcinogenesis Online).
Table IV.
Analysis of SNP associations in the six significant sex hormone pathway genes (P < 0.05) and risk of ESCC by sex
| Gene | Gene.P◊ | Most significant SNP | Male | Female | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MAF cases/control | OR | 95% CI | P | MAF cases/control | OR | 95% CI | P | P | |||
| SULT2B1 | 0.013 | rs4149455 | 0.38/0.44 | 0.80 | 0.67–0.91 | 0.002 | 0.37/0.40 | 0.90 | 0.71–1.04 | 0.120 | 0.516 |
| CYP1B1 # | 0.019 | rs9341266 | 0.09/0.06 | 1.70 | 1.23–2.31 | 0.001 | 0.08/0.06 | 1.38 | 0.95–2.01 | 0.090 | 0.484 |
| CYP3A7 | 0.030 | rs2005548 | 0.28/0.25 | 1.16 | 0.98–1.37 | 0.084 | 0.30/0.26 | 0.84 | 0.70- 100 | 0.053 | 0.734 |
| CYP3A5 | 0.034 | rs17161780 | 0.28/0.26 | 1.20 | 1.05–1.45 | 0.001 | 0.31/0.25 | 1.10 | 0.94–1.36 | 0.190 | 0.631 |
| SHBG | 0.037 | rs727428 | 0.37/0.40 | 0.89 | 0.75–1.04 | 0.137 | 0.38/0.42 | 0.84 | 0.70–1.00 | 0.053 | 0.907 |
| CYP11A1 # | 0.038 | rs2277604 | 0.08/0.04 | 1.90 | 1.34–2.69 | 0.0003 | 0.07/0.07 | 1.10 | 0.73–1.53 | 0.770 | 0.034 |
ORs and P values were obtained for the most significantly associated SNPs in SULT2B1, CYP1B1, CYP3A7, CYP3A5, SHBG and CYP11A1 following stratification of the combined study population by sex. CYP1B1 rs9341266 and CYP11A1 rs2277604 were analyzed using a dominant model (#), whereas the remaining SNP ORs are per allele ORs.
Tobacco and alcohol stratification and gene-based pathway analysis
Although tobacco and alcohol are not major risk factors for ESCC in our high-risk Chinese population, we examined the most significant SNP in each of the six significant genes identified (i.e. SULT2B1, CYP1B1, CYP3A7, CYP3A5, SHBG and CYP11A1) by smoking status and alcohol use. Because few women in our study populations smoked or drank alcohol, we examined the SNP associations with ESCC risk in men only and found no variation in risk by either smoking or drinking status in men (data not shown).
Discussion
The 2.4:1 male:female ratio for ESCC incidence worldwide suggests a potential role for sex hormones in ESCC etiology (2). In particular, previous data have reported that both estrogen and androgen receptors are expressed in ESCC in vivo and that these hormones altered growth of ESCC cells in vitro. These data led investigators to suggest a possible role for sex hormones in the development of ESCC (22–28). A pooled analysis of three European case–control studies also found that ever use of hormone therapy was associated with a reduced risk of ESCC in women (OR = 0.32, 95% CI: 0.09–1.13) (20). Consistent with these findings, independent reports by Freedman et al. (18) and Bodelon et al. (19) from cohorts in the USA have suggested that this inverse association with ESCC was limited to users of estrogen plus progestin. In a high-ESCC incidence area in China, serum estradiol levels in ESCC cases were significantly reduced compared with age-matched, healthy subjects from both high- and low-ESCC incidence areas. Furthermore, control subjects from the high-ESCC incidence area had lower levels of estradiol than control subjects from the low-ESCC incidence area, suggesting that estradiol may be associated with reduced risk of ESCC (21).
Although the biological mechanism by which estrogen and/or androgens might affect ESCC risk and/or development has yet to be elucidated, polymorphisms in specific sex hormone metabolic genes have been associated with variation in the levels of gene products and, in turn, circulating sex hormone levels (and/or metabolites) (37–39). Evidence also suggests that polymorphisms in such genes can modify hormone therapy-associated colorectal cancer risk in postmenopausal women (40). Thus, functionally relevant SNPs in sex hormone metabolic genes may alter exposure to both endogenous and exogenous hormones and thereby affect the risk of ESCC. Previous studies have reported inconsistent associations between specific polymorphisms in sex hormone metabolic genes such as GSTP1 (rs1695), SULT1A1 (rs10420278), COMT (rs4680) and AR (CAG/GGC tandem repeats) (14) and ESCC risk, but a meta-analysis of studies investigating CYP1A1 rs1048943 (1506 A>G) and ESCC found overall significant summary ORs of 2.52 (95% CI: 1.62–3.91) and 1.44 (95% CI: 1.17–1.78) for heterozygous and homozygous genotypes, respectively (15). Our study, however, failed to replicate these previous findings.
To our knowledge, this is the first study to comprehensively assess the association between common polymorphisms in key genes involved in the sex hormone metabolic pathway with risk of ESCC. We did not observe a pathway-based association with ESCC when all 51 genes in the sex hormone metabolic pathway were considered together; however, significant associations were observed for six genes in the pathway. The strongest gene-based association observed was for SULT2B1. The human SULT2B1 gene is a member of the cytosolic SULT superfamily and has two isoforms: SULT2B1a and SULT2B1b, both of which are derived from a single gene (SULT2B1). SULT2B1a preferentially catalyzes sulfonation of pregnenolone and DHEA, and SULT2B1b functions as a selective cholesterol sulfotransferase (41). Two SNPs in SULT2B1 were associated with risk of ESCC. The variant allele of rs4149455, which is an intronic polymorphism (IVS6-436 C>T), was associated with reduced risk of ESCC (per allele OR: 0.81, 95% CI: 0.72–0.91, P = 0.007), as was the variant T allele of rs1052131 which is a synonymous polymorphism (g.Ex7+122C>T; p.D316D) that maps to both variant isoforms (per allele OR: 0.84, 95% CI: 0.70–0.99, P = 0.048).
Cytochrome P450 1B1 (CYP1B1) is an important estrogen metabolizing enzyme and several variants of the gene are known to have higher catalytic activity than the wild-type enzyme in converting estrogen to 4-hydroxy estrogens and inducing DNA damage (42). For CYP1B1, we found evidence of a strong association between ESCC risk and a single SNP rs9341266 (G/A) in the 3′ untranslated region (per allele OR: 1.45, 95% CI: 1.16–1.82, P = 0.001).
The four CYP3A (A4, A5P2, A7 and A5) genes are localized in tandem on chromosome 7q21–q22.1 (43). In this study, three CYP3A SNPs associated with increased risk of ESCC (rs4646450, rs776746 and rs2005548) were in strong LD (r 2 ≥ 0.8) across the CYP3A cluster. CYP3A enzymes metabolize various steroids, such as progesterone, estradiol, testosterone and corticosterone (44). CYP3A activity also varies (>30-fold) widely between ethnic populations (45). The G allele (7786G>A) of rs17161780 in CYP3A5 is a common allele in Chinese and has previously been referred to as the mutant CYP3A5*3 allele, which encodes an aberrantly spliced mRNA with a premature stop codon (44). Specifically, only individuals with at least one variant A allele produce high levels of the CYP3A5 enzyme (44). In our control population, we found that the frequency of the G allele was 0.74. In a South African study, individuals homozygous for the mutant allele (GG) showed decreased ESCC risk, whereas heterozygotes (GA genotype) had increased risk (46). Evidence in our Chinese population also suggests that individuals heterozygous (GA) for rs17161780 have an increased risk of ESCC and this risk is further increased in individuals homozygous for the A allele (OR: 1.52, 95% CI: 1.12–2.05, P = 0.006). Three other CYP3A5 SNPs (rs4646453, rs4646450 and rs776746) were also associated with increased risk of ESCC, but were in strong LD (r 2 ≥ 0.8) with rs17161780.
The sex hormone-binding globulin (SHBG) glycoprotein binds to and transports both androgens and estrogens in the blood, thus altering bioavailability and activity at the tissue level. In this study, the C allele of rs727428 had the strongest association with reduced ESCC risk of all SHBG SNPs tested. SHBG rs727428 (T allele) has previously been associated with decreased SHBG levels in postmenopausal Caucasian women (47), suggesting Chinese individuals with the C variant may have high levels of SHBG. Previously, we reported rs2955617 as part of an LD block (CACCC haplotype) of the SHBG coding region that was associated with lower gastric cancer risk in Caucasians (48).
CYP11A1 is a key rate-limiting enzyme involved in steroidogenesis and the conversion of cholesterol to pregnenolone. Pregnenolone is further catalyzed by other steroidogenic enzymes (e.g. CYP17, CYP21, CYP11B1, CYP11B2, HSD3B and CYP19) to various steroid products including mineralocorticoids, glucocorticoids and sex hormones (31). In our study, CYP11A1 rs2277604 (T allele) was associated with increased risk of ESCC.
Worldwide, ESCC is considerably more common among men than women (2). In order to evaluate whether the main associations found in our study varied by sex in our high-risk Chinese population, we examined the overall sex hormone metabolic pathway, three subpathways and six significant genes separately in males and females. The most significant SNP in one of the six genes, rs2277604 in CYP11A1, showed a significantly stronger association with ESCC risk in males than females. Also in males, the androgen synthesis subpathway was significantly associated with ESCC risk.
Given that CYP11A1 catalyzes the first rate-limiting step in sex hormone biosynthesis from cholesterol, it is plausible that one or more SNPs in CYP11A1 could result in altered levels of sex steroid hormones and thereby affect risk of ESCC. However, rs2277604, which localizes to the 3′ gene region of CYP11A1, also colocalizes to exon 20b of another gene called coiled-coil domain containing 33 (CCDC33), resulting in a missense change at codon 473 (Arg->Leu). Recently, the CCDC33 protein, also called FAVINE, was reported to be a putative peroxisomal protein (49) secreted by vascular and adipose tissues that was regulated both hormonally and nutritionally (50). Although our data suggest that the association of CYP11A1 rs2277604 with ESCC risk differs by sex, this association could potentially be driven by CCDC33. In addition, the sex difference in effect size for CYP11A1 rs2277604 was apparent in both study populations, but was significant only in the Shanxi study. Thus, our findings for rs2277604 should be interpreted with caution until further confirmed in additional studies.
Controls were more likely to drink and use tobacco than cases, which supports previous findings that these exposures are not major risk factors for ESCC in the high-risk populations studied here. In addition, neither smoking nor alcohol confounded our genotypic findings. However, CYP and SULT enzymes also play key roles in the oxidative metabolism of other endogenous and exogenous compounds as well as the removal of lipophilic foreign chemicals. Therefore, it is possible that our findings regarding SULT2B1, CYP1B1, CYP3A5, CYP3A4 and CYP11A1 could reflect alternative functions and/or potential involvement of these gene products in the metabolism of other compounds (41,44).
To our knowledge, this is the first study to comprehensively investigate the role of genetic variation in sex hormone metabolic genes and risk of ESCC. The relatively large number of ESCC cases in this study allowed us to assess risk with good power, including estimation of sex-specific associations. We also benefited from detailed information on other potential risk factors (e.g. tobacco, alcohol and family history of UGI cancer). Our results could, of course, be influenced by unmeasured or unknown confounders. Also this study was conducted in high-risk Chinese populations, so generalizability to other populations remains to be determined. Another limitation is the use of tag SNPs that are themselves unlikely to be disease-related SNPs but are assumed to be in LD with causal variants. To address this limitation, we relied mainly on the robust gene-based ARTP method to confirm associations with risk of ESCC. In conclusion, genetic variations in six sex hormone metabolic pathway genes were associated with ESCC risk, suggesting a potential role for sex hormones in the etiology of ESCC. Further studies are merited to replicate these findings in other populations and to investigate functional alterations in these genes.
Supplementary material
Supplementary Tables 1–6 can be found at http://carcin.oxfordjournals.org/
Funding
Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics; Cancer Prevention Fellowship Program, Division of Cancer Prevention, National Cancer Institute, Bethesda, USA (to P.L.H.); Health and Social Care, Northern Ireland, UK (to P.L.H.).
Conflict of Interest Statement: None declared.
Supplementary Material
Glossary
Abbreviations:
- AR
androgen receptor
- ARTP
adaptive rank truncated product
- CI
confidence interval
- ER
estrogen receptor
- ESCC
esophageal squamous cell carcinoma
- LD
linkage disequilibrium
- NIT
Nutrition Intervention Trial
- OR
odds ratio
- SHBG
sex hormone-binding globulin
- SNP
single-nucleotide polymorphisms
- UGI
upper gastrointestinal.
References
- 1. Parkin D.M., et al. (2005). Global cancer statistics, 2002. CA. Cancer J. Clin., 55, 74–108 [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J., et al. (2010). Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer., 127, 2893–2917 [DOI] [PubMed] [Google Scholar]
- 3. Qiao Y.L., et al. (2009). Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. J. Natl. Cancer Inst., 101, 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO and IARC World Cancer Report (2008). WHO, IARC; Lyon: [Google Scholar]
- 5. Lee C.H., et al. (2007). Carcinogenetic impact of alcohol intake on squamous cell carcinoma risk of the oesophagus in relation to tobacco smoking. Eur. J. Cancer., 43, 1188–1199 [DOI] [PubMed] [Google Scholar]
- 6. Pandeya N., et al. (2009). Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology, 136, 1215–1224 [DOI] [PubMed] [Google Scholar]
- 7. Tran G.D., et al. (2005). Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int. J. Cancer., 113, 456–463 [DOI] [PubMed] [Google Scholar]
- 8. Wei W.Q., et al. (2005). Risk factors for oesophageal squamous dysplasia in adult inhabitants of a high risk region of China. Gut, 54, 759–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu N., et al. (1992). Familial aggregation of oesophageal cancer in Yangcheng County, Shanxi Province, China. Int. J. Epidemiol., 21, 877–882 [DOI] [PubMed] [Google Scholar]
- 10. Gao Y., et al. (2011). Risk factors for esophageal and gastric cancers in Shanxi Province, China: a case-control study. Cancer Epidemiol., 35, e91–e99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu Y., et al. (1993). Retrospective cohort study of risk-factors for esophageal cancer in Linxian, People’s Republic of China. Cancer Causes Control., 4, 195–202 [DOI] [PubMed] [Google Scholar]
- 12. Carter C.L., et al. (1992). Segregation analysis of esophageal cancer in 221 high-risk Chinese families. J. Natl. Cancer Inst., 84, 771–776 [DOI] [PubMed] [Google Scholar]
- 13. Zhang W., et al. (2000). Segregation analysis of esophageal cancer in a moderately high-incidence area of northern China. Am. J. Hum. Genet., 67, 110–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hiyama T., et al. (2007). Genetic polymorphisms and esophageal cancer risk. Int. J. Cancer., 121, 1643–1658 [DOI] [PubMed] [Google Scholar]
- 15. Yang C.X., et al. (2005). Phase I/II enzyme gene polymorphisms and esophageal cancer risk: a meta-analysis of the literature. World J. Gastroenterol., 11, 2531–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang H., et al. (2010). The high incidence of esophageal cancer in parts of China may result primarily from genetic rather than environmental factors. Dis. Esophagus., 23, 392–397 [DOI] [PubMed] [Google Scholar]
- 17. Abnet C.C., et al. (2010). A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nat. Genet., 42, 764–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Freedman N.D., et al. (2010). The association of menstrual and reproductive factors with upper gastrointestinal tract cancers in the NIH-AARP cohort. Cancer, 116, 1572–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bodelon C., et al. (2011). Hormonal factors and risks of esophageal squamous cell carcinoma and adenocarcinoma in postmenopausal women. Cancer Prev. Res. (Phila)., 4, 840–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gallus S., et al. (2001). Oesophageal cancer in women: tobacco, alcohol, nutritional and hormonal factors. Br. J. Cancer., 85, 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Q.M., et al. (2011). Relevance of serum estradiol and estrogen receptor beta expression from a high-incidence area for esophageal squamous cell carcinoma in China. Med. Oncol., 28, 188–193 [DOI] [PubMed] [Google Scholar]
- 22. Nozoe T., et al. (2007). Significance of immunohistochemical expression of estrogen receptors alpha and beta in squamous cell carcinoma of the esophagus. Clin. Cancer Res., 13, 4046–4050 [DOI] [PubMed] [Google Scholar]
- 23. Kalayarasan R., et al. (2008). Estrogen and progesterone receptors in esophageal carcinoma. Dis. Esophagus., 21, 298–303 [DOI] [PubMed] [Google Scholar]
- 24. Matsuoka H., et al. (1990). Estradiol sensitivity test using contact-sensitive plates of confluent BALB/c 3T3 cell monolayers. Cancer Res., 50, 2113–2118 [PubMed] [Google Scholar]
- 25. Rashid F., et al. (2010). Probing the link between oestrogen receptors and oesophageal cancer. World J. Surg. Oncol., 8, 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ueo H., et al. (1990). Inhibitory effects of estrogen on the growth of a human esophageal carcinoma cell line. Cancer Res., 50, 7212–7215 [PubMed] [Google Scholar]
- 27. Yamashita Y., et al. (1989). Detection of androgen receptors in human esophageal cancer. Jpn. J. Surg., 19, 195–202 [DOI] [PubMed] [Google Scholar]
- 28. Tihan T., et al. (2001). Evidence of androgen receptor expression in squamous and adenocarcinoma of the esophagus. Anticancer Res., 21,(4B)3107–3114 [PubMed] [Google Scholar]
- 29. Taylor P.R., et al. (1994). Prevention of esophageal cancer: the nutrition intervention trials in Linxian, China. Linxian Nutrition Intervention Trials Study Group. Cancer Res., 54,(7 Suppl)2029s–2031s [PubMed] [Google Scholar]
- 30. Li B., et al. (1993). Linxian nutrition intervention trials. Design, methods, participant characteristics, and compliance. Ann. Epidemiol., 3, 577–585 [DOI] [PubMed] [Google Scholar]
- 31. Miller W.L. (2008). Steroidogenic enzymes. Endocr. Dev., 13, 1–18 [DOI] [PubMed] [Google Scholar]
- 32. Payne A.H., et al. (2004). Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev., 25, 947–970 [DOI] [PubMed] [Google Scholar]
- 33. Li L., et al. (2010). Genetic variation in the estrogen metabolic pathway and mammographic density as an intermediate phenotype of breast cancer. Breast Canc. Res., 12, R19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang H.P., et al. (2010). Common genetic variation in the sex hormone metabolic pathway and endometrial cancer risk: pathway-based evaluation of candidate genes. Carcinogenesis, 31, 827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aoki K.F., et al. (2005). Using the KEGG database resource. Curr. Protoc. Bioinformatics., Chapter 1, [DOI] [PubMed] [Google Scholar]
- 36. Yu K., et al. (2009). Pathway analysis by adaptive combination of P-values. Genet. Epidemiol., 33, 700–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang J., et al. (2010). Association of genetic variations in aromatase gene with serum estrogen and estrogen/testosterone ratio in Chinese elderly men. Clin. Chim. Acta., 411, 53–58 [DOI] [PubMed] [Google Scholar]
- 38. Eriksson A.L., et al. (2009). Genetic variations in sex steroid-related genes as predictors of serum estrogen levels in men. J. Clin. Endocrinol. Metab., 94, 1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vanbillemont G., et al. (2009). Polymorphisms of the SHBG gene contribute to the interindividual variation of sex steroid hormone blood levels in young, middle-aged and elderly men. Clin. Endocrinol. (Oxf)., 70, 303–310 [DOI] [PubMed] [Google Scholar]
- 40. Rudolph A., et al. (2011). Modification of menopausal hormone therapy-associated colorectal cancer risk by polymorphisms in sex steroid signaling, metabolism and transport related genes. Endocr. Relat. Cancer., 18, 371–384 [DOI] [PubMed] [Google Scholar]
- 41. Ji Y., et al. (2007). Human hydroxysteroid sulfotransferase SULT2B1 pharmacogenomics: gene sequence variation and functional genomics. J. Pharmacol. Exp. Ther., 322, 529–540 [DOI] [PubMed] [Google Scholar]
- 42. Hanna I.H., et al. (2000). Cytochrome P450 1B1 (CYP1B1) pharmacogenetics: association of polymorphisms with functional differences in estrogen hydroxylation activity. Cancer Res., 60, 3440–3444 [PubMed] [Google Scholar]
- 43. Lee S.J., et al. (2005). Functionally defective or altered CYP3A4 and CYP3A5 single nucleotide polymorphisms and their detection with genotyping tests. Pharmacogenomics, 6, 357–371 [DOI] [PubMed] [Google Scholar]
- 44. Lamba J.K., et al. (2002). Genetic contribution to variable human CYP3A-mediated metabolism. Adv. Drug Deliv. Rev., 54, 1271–1294 [DOI] [PubMed] [Google Scholar]
- 45. Kuehl P., et al. (2001). Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat. Genet., 27, 383–391 [DOI] [PubMed] [Google Scholar]
- 46. Dandara C., et al. (2005). CYP3A5 genotypes and risk of oesophageal cancer in two South African populations. Cancer Lett., 225, 275–282 [DOI] [PubMed] [Google Scholar]
- 47. Thompson D.J., et al. (2008). Identification of common variants in the SHBG gene affecting sex hormone-binding globulin levels and breast cancer risk in postmenopausal women. Cancer Epidemiol. Biomarkers Prev., 17, 3490–3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Freedman N.D., et al. (2009). Polymorphisms in estrogen- and androgen-metabolizing genes and the risk of gastric cancer. Carcinogenesis, 30, 71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kaczmarek K., et al. (2009). Ccdc33, a predominantly testis-expressed gene, encodes a putative peroxisomal protein. Cytogenet. Genome Res., 126, 243–252 [DOI] [PubMed] [Google Scholar]
- 50. Kobayashi S., et al. (2010). Identification of a new secretory factor, CCDC3/Favine, in adipocytes and endothelial cells. Biochem. Biophys. Res. Commun., 392, 29–35 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.