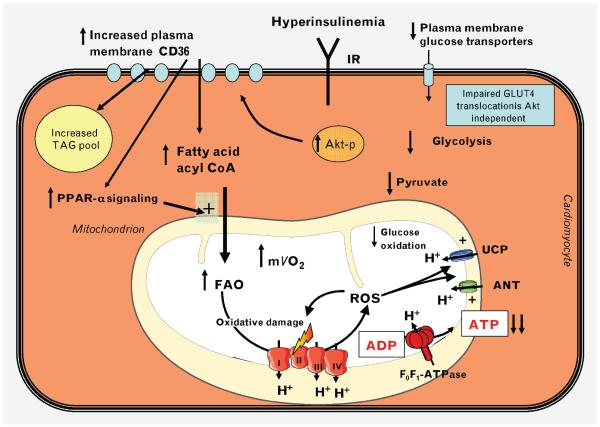
Figure 2.
Mechanisms leading to increased myocardial fatty acid oxidation in insulin-resistant states. In the evolution of diet-induced obesity, or in type 2 diabetes, hyperinsulinemia activates insulin receptors (IR) and Akt (protein kinase B), leading to increased plasma membrane translocation of CD36, which leads to increased fatty acid uptake. Decreased expression and translocation of glucose transporter-4 (GLUT4) in insulin resistance leads to decreased glucose uptake and decreased glycolysis, which further increase fatty acid utilization. Reduced GLUT4 translocation precedes significant downregulation of insulin signal transduction to Akt. Mechanisms for reduced GLUT4 translocation are incompletely understood. Increased lipid availability activates PPARa, which leads to increased expression of proteins involved in fatty acid utilization, and increased pyruvate dehydrogenase kinase-4 (PDK4), which increase fatty acid oxidation (FAO) and decrease glucose oxidation, respectively. Increased FAO is associated with increased myocardial oxygen consumption (mVO2). As insulin resistance progresses and diabetes ensues, reactive oxygen species (ROS)-mediated mitochondrial uncoupling develops, which further increases mVO2, decreases ATP generation, and decreases cardiac efficiency. I–IV, Mitochondrial electron transport chain complexes I-IV; Akt-p, phospho-Akt/protein kinase B; ANT, adenine nucleotide translocase; CoA, coenzyme A; TAG, triacylglycerol; UCP, uncoupling proteins 2 and 3.