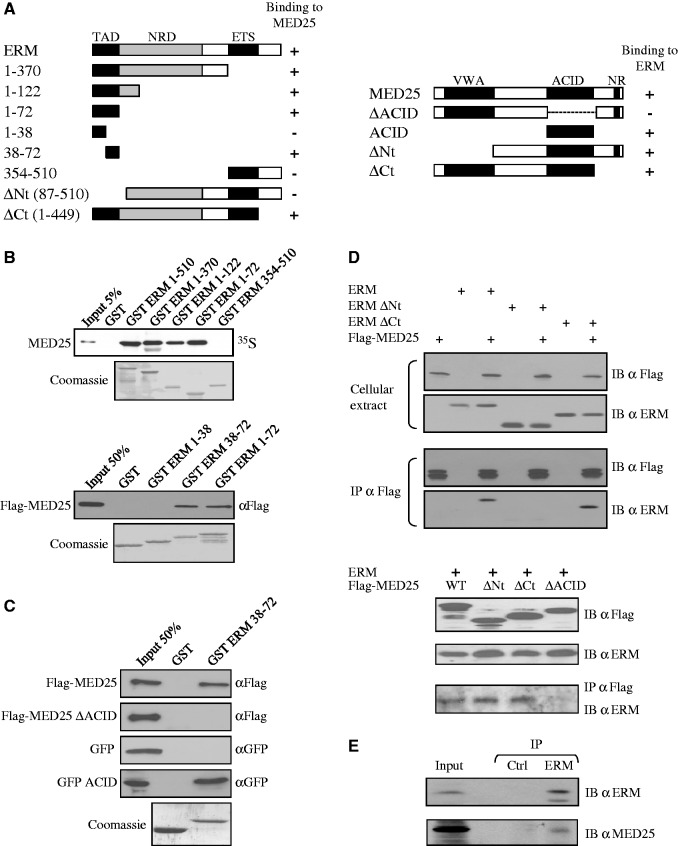
Figure 1.
ERM binds to MED25. (A) Schematic summary of the interaction between ERM and MED25 proteins. The N-terminal TAD of ERM interacts with the ACID of MED25. NR: Nuclear receptor box. Numbers refer to amino acid. (B) Deletion analysis of ERM shows that the N-terminal 38–72 domain is sufficient for binding MED25 in vitro. GST fusion proteins of the indicated ERM fragments were used to assess the binding to full-length rabbit reticulocyte lysate in vitro generated Flag-MED25. Binding was detected by autoradiography (upper panel) or immunoblotting with anti-Flag (bottom panel). An SDS gel stained with Coomassie showing the expression of the GST fusion proteins is shown. (C) Deletion analysis of MED25 shows that the ACID domain is sufficient for binding to ERM 38–72 in vitro. GST and GST-ERM 38–72 were incubated with the indicated MED25 fragments produced in reticulocyte lysate. Binding was detected by immunoblotting with anti-Flag or anti-GFP. (D) Co-immunoprecipitation of MED25 with ERM. Flag-MED25 and wild-type or mutants ERM (upper panel) or mutants Flag-MED25 together with full-length ERM (bottom panel) were expressed in RK13 cells. Cellular extracts were immunoprecipitated with anti-Flag antibody (IP α Flag) and immunoblotted with anti-Flag (IB α Flag) and anti-ERM (IB α ERM) antibodies. Aliquots of the same extracts were analysed with the same antibodies to detect exogenous proteins (cellular extract). (E) Interaction between endogenous proteins. MDA-MB 231 nuclear extracts were subjected to immunoprecipitation with anti-Gal4 DBD (IP Ctrl) or anti-ERM (IP ERM) antibodies. Interactions were detected by western blot using polyclonal anti-MED25 (IB α MED25) or polyclonal anti-ERM (IB α ERM).