Figure 2.
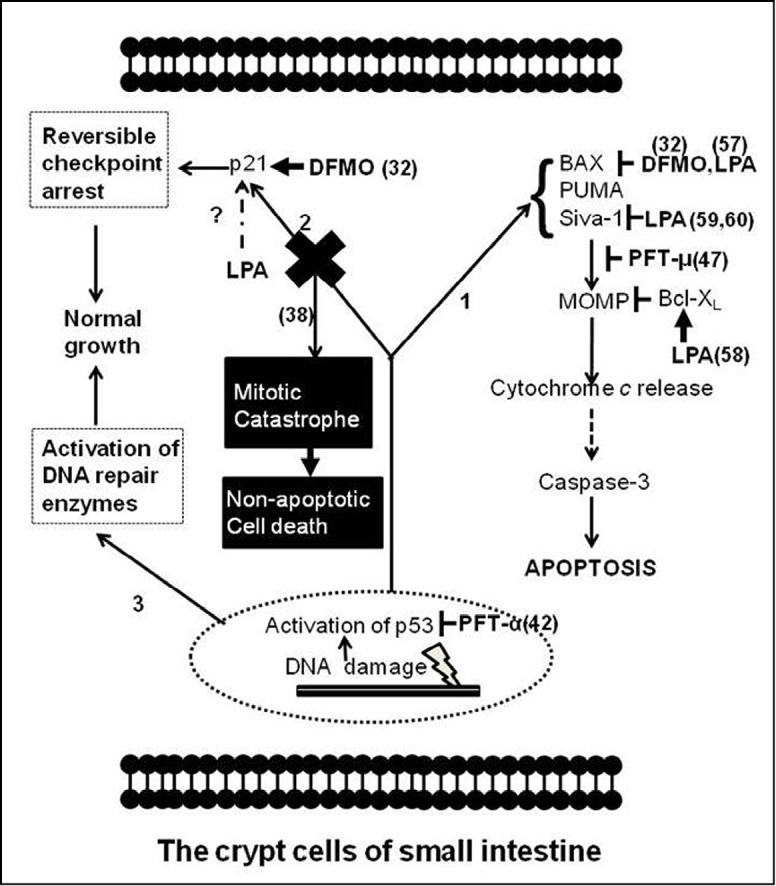
Schematic representation of p53 signaling in response to anticancer drug treatment and radiation therapy in crypt cells of the small intestine and action sites of 3 molecules (PFT, DFMO, and LPA). In response to DNA damage, p53 accumulation leads to the activation of three pathways involved in (1) apoptosis, (2) cell cycle arrest, and (3) DNA repair. The transcriptional activity of p53 increases the expression levels of proteins responsible for the three pathways, such as p21 and Bax. The cytosol function of p53 also directly induces the MOMP. PFT-α inhibits the transcriptional activity of p53 [28], whereas PFT-µ binds p53 to attenuate the binding affinity of anti-apoptotic proteins, such as Bcl-XL[29]. DFMO increases the expression of p21 but inhibits that of Bax [30]. LPA blocks the translocation of Bax from cytosol to mitochondria [31], accelerates the protein degradation of pro-apoptotic Siva-1 [32,33], and increases the protein expression of Bcl-XL [34]. The effect of LPA on p21 in response to DNA damage is currently unknown. In some conditions, such as high-dose radiation for cancer treatment, blocking p53 (such as in p53 KO mice) in crypts of the small intestine leads to mitotic catastrophe, a type of cell death occurring during mitosis, as a result of DNA damage [35]. Inhibiting the p53-mediated p21 pathway is a major mechanism responsible for mitotic catastrophe in cells with unrepaired DNA. The p53-mediated cell cycle arrest pathway is hypothesized to offer cells a time window to process DNA repair in response to DNA damage. The numbers within parentheses indicate the sources of these findings. Detailed mechanisms related to apoptosis have been reviewed elsewhere [12-14].
