Table 4.
Dose-response and solubility of the 12 kinase inhibitors.
| Structure | Name | ADP production IC50 (μM) | ADP processing IC50 (μM) | Luminescence detection IC50 (μM) | Solubility | Use/Therapy |
|---|---|---|---|---|---|---|
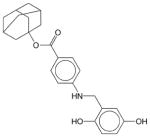
|
Adaphostin | N.E.1 | N.E. | N.E. | Soluble | BCR-ABL inhibitor; adamantyl ester of tyrphostin AG 957. Shows selective activity against chronic and acute myeloid leukemia. Has not been approved as a drug. |
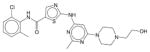
|
Dasatinib | N.E. | N.E. | N.E. | Soluble | BCR-ABL inhibitor. A cancer drug Sprycel for use in patients with chronic myelogenous leukemia after imatinib treatment and Philadelphia chromosome-positive acute lymphoblastic leukemia. |
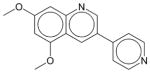
|
DMPQ | N.E. | N.E. | N.E. | Soluble | PDGFRβ inhibitor. Has not been approved as a drug. |
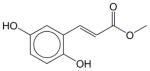
|
Erbstatin | N.E. | N.E. | N.E. | Soluble | EGFR inhibitor. Has not been approved as a drug. |
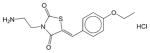
|
Erk inhibitor | N.E. | N.E. | N.E. | Soluble | Extracellular signal-regulated kinase inhibitor. Has not been approved as a drug. |
|
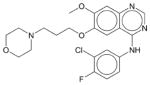
Gefitinib | N.E. | N.E. | N.E. | Soluble | EGFR inhibitor. A cancer drug Iressa for use in patients with non-small cell lung cancer with EGFR mutations in Europe, Asia, and Australia. |
|
|
Gleevec | N.E.1 | N.E. | N.E. | Soluble | BCR-ABL inhibitor. A cancer drug Imatinib for use in patients with chronic myelogeneous leukemia and gastrointestinal tumors. |
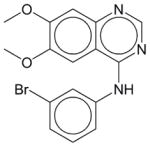
|
PD 153035 | N.E. | N.E. | N.E. | Soluble | EGFR inhibitor, with an IC50 of 25 pM. Inhibits other purified tyrosine kinases only at micromolar or higher concentrations. Has not been approved as a drug. |
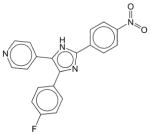
|
PD 169316 | N.E. | N.E. | N.E. | Soluble | A selective inhibitor of p38 MAPK; induces apoptosis of neuronal and non-neuronal cells deprived of specific trophic factors. Has not been approved as a drug. |
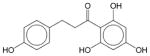
|
Phloretin | N.E. | N.E. | N.E. | Soluble | Inhibits glucose transmembrane transport and protein kinase C activity. Induces growth inhibition and apoptosis in human leukemia and colon cancer cells. Has not been approved as a drug. |
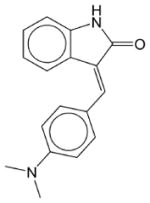
|
SU 4312 | N.E. | N.E. | N.E. | Soluble | VEGFR 1/2 and PDGFR inhibitor. Has not been approved as a drug. |
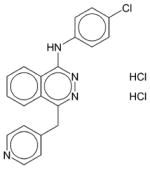
|
Vatalanib | N.E. | N.E. | N.E. | Soluble | VEGF inhibitor; PTK787. Inhibits angiogenesis. Phase III trials in people with metastatic colorectal cancer. Has not been approved as a drug. |
N.E., no effect.
