Abstract
Platelet-activating factor (PAF) is a naturally occurring phospholipid that mediates diverse effects such as physiological and pathological inflammation, immunosuppression, and cancer. Several lines of evidence support both positive and negative roles for PAF in carcinogenesis. PAF stimulates cell growth, oncogenic transformation and metastasis, but can also limit proliferation and induce apoptosis. The biological context and microenvironment appear to define whether PAF has pro- or anti-carcinogenic effects. To investigate the role of exacerbated PAF signaling in colon cancer, we conducted cell-based and in vivo studies using genetically engineered mice lacking expression of phospholipase A2 group 7 (PLA2G7), an enzyme that specifically metabolizes PAF and structurally related glycerophospholipids. Absence of Pla2g7 robustly decreased intestinal polyposis and colon tumor formation in Apc Min/+ mice, suggesting an anti-tumorigenic role for PAF in settings characterized by aberrant function of the tumor suppressor Adenomatous polyposis coli (Apc). In colonic epithelial cells, exposure to a PAF analog led to de-phosphorylation of Akt at serine-473 and induction of apoptosis. The mechanism of this response involved formation of a complex between β-arrestin 1 and the Akt phosphatase PHLPP2, and activation of the intrinsic pathway of apoptosis. Our results suggest that strategies based on inhibiting PLA2G7 activity or increasing PAF-mediated signaling, hold promise for the treatment of intestinal malignancies that harbor mutations in APC.
Keywords: Colon cancer, Akt, phosphatase, apoptosis, platelet-activating factor
Introduction
Colorectal cancer is one of the leading causes of cancer death in the Western world. More than 80% of colorectal cancers harbor mutations in the Adenomatous polyposis coli (APC) gene (1). A number of studies suggest that the generation of an inflammatory microenvironment promotes tumor development (2). Additionally, the risk of developing colorectal cancer is related to the duration, extent, and severity of inflammatory disease (3). Platelet-activating factor (PAF, 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) is a pro-inflammatory phospholipid that plays important roles in the control of immune cell functions (4). PAF evokes growth factor-like signals in many cell types (5) and mediates its effects primarily through a G-protein-coupled receptor [PAFR, (6)]. Over-expression of PAFR results in development of melanocytic tumors (7) and promotes ovarian cancer (8). PAF is metabolized to lyso-PAF by specific intracellular and secreted, calcium-independent, phospholipase A2 (PLA2) activities known as PAF acetylhydrolases. The secreted, plasma form of this family of enzymes is also known as phospholipase A2 group 7 (PLA2G7).
Numerous studies have shown that in settings of intestinal inflammation, PAF plays a detrimental role. Excess PAF generated during acute bowel injury actively contributes to the development of ischemic colitis and inflammatory bowel diseases (9) by increasing vascular permeability, inflammation, and chronic epithelial damage (10, 11). Enhanced PAF signaling by deletion of Pla2g7 increased the severity of inflammation in a neonatal model of necrotizing enterocolitis (12). Similarly, in models of melanoma and breast cancer, PAF accumulation is thought to enhance tumorigenesis owing to its role in oncogenic transformation (5), metastasis (13), immuno-suppression (14) and angiogenesis (15).
Although most studies suggest a tumor-promoting role for PAF, the biological consequences associated with enhanced signaling via PAF/PAFR are highly dependent on the biological context and the levels of PAF and/or other PAFR ligands. PAF is synthesized in small intestinal tissues (16) and has homeostatic functions in the normal bowel, such as regulation of vectorial ion transport and mucosal permeability (17, 18). Thus, disruption of homeostatic PAF signaling may have untoward effects in defined settings. While deletion of Pla2g7 increased inflammation in a neonatal model of necrotizing enterocolitis (see above), mortality rates before 24 h of life were significantly lower in Pla2g7/−/−pups compared with wild-type controls (12). Thus, both PLA2G7 and PAF appear to play multiple roles depending on developmental stage, degree of intestinal damage and extent of inflammation. Clearly, signaling events mediated by the PAF/PAFR/PLA2G7 axis are highly dependent on the environment and ligand concentration, and need to be defined in specific biological settings.
In the intestine, exposure to micromolar levels of PAF has anti-tumorigenic roles as judged by its ability to inhibit proliferation, induce differentiation and suppress the malignant phenotype of human colon carcinoma cells (4). PAF has been reported to mediate apoptosis through both the extrinsic (i.e., Fas/FasL) and intrinsic (mitochondrial) apoptotic pathways (19, 20). Interestingly, lower levels of PAF were detected in tumor tissues from patients with advanced forms of colon cancer (Duke stages 3 and 4) compared with tumors evaluated at earlier disease stages (21). Plasma and tumor tissues from patients diagnosed with colon cancer have been reported to express 50% higher levels of PLA2G7 activity compared with samples from apparently healthy subjects (21, 22). Similar trends were observed in tissue extracts from liver metastases of colorectal cancers (23). Expression of PLA2G7 mRNA is up-regulated by combined mutation of Ras and p53 in young adult murine colon cells, and silencing PLA2G7 expression reduces the growth of these cells in vivo (24). PLA2G7 has been reported to be a cooperation response gene that plays a role in malignant cell transformation (24).
The Apc Min/+ mouse model of multiple intestinal neoplasia is commonly utilized to identify mediators and characterize mechanisms that govern initiation, establishment, and progression of intestinal tumors (25). Apc Min/+ mice are heterozygous for a mutation that results in a truncated form of Apc; these animals develop spontaneous adenomas in the small intestine and fewer adenomas in the colon (25). Their propensity to form adenomas is similar to that of Familial Adenomatous Polyposis patients who harbor mutations in the tumor suppressor APC. These subjects develop multiple intestinal adenomas that progress into malignant adenocarcinomas (26). Most human colorectal cancers also harbor mutations in APC that are thought to impact key processes affecting tumorigenesis, such as differentiation, Wnt/β-catenin signaling, and retinoic acid biosynthesis (26). In this study, we investigated whether altered signaling via the PAF/PAFR axis affects events relevant to colon carcinogenesis in settings characterized by deregulated APC function. To accomplish this, we conducted studies in Apc Min/+ mice crossed to animals that lack expression of PLA2G7, one of the key enzymes involved in PAF metabolism. To address mechanistic issues, we investigated responses to PAF treatment in human colon cancer cells. Our data show that genetic deletion of Pla2g7 reduces intestinal polyposis through PAFR-mediated de-phosphorylation of Akt by the phosphatase PHLPP2, and induction of apoptosis via the intrinsic pathway.
Materials and Methods (see also Supplementary Material section)
Cell culture and treatment
Human colorectal adenocarcinoma cell lines HT-29, Caco-2, Lovo, SW-480 cells, and colorectal carcinoma HCT-116 cells were obtained from American Type Culture Collection where they are regularly tested for viability, recovery, growth, morphology, and isoenzymology. Human colonic adenocarcinoma HCA-7 cells were from Life Technologies Corporation Collection. All lines were maintained at 37°C in a 5% CO2 atmosphere, in Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum and penicillin/streptomycin. Phospholipids (i.e, PAF, cPAF, and lyso-PAF) used to stimulate cells were dried under a stream of nitrogen and were re-suspended in 1 mg/ml fatty-acid free bovine serum albumin before addition to the cells.
Assessment of cellular proliferation
HT-29 cells seeded in 96-well plates were allowed to grow in the presence of 10% FBS, and were then placed in 0–0.1% FBS-containing medium, as indicated in the figure legends. The cells were exposed to fresh media containing cPAF and BN-52021 (where indicated) every 24 h. After a total of 72 hours at 37°C, we assessed cellular proliferation using the XTT assay, following the manufacturer’s specifications. Briefly, each well was supplemented with XTT labeling mixture and the plates then were incubated for 4 h at 37°C. Absorbance at 450 nm and 650 nm was assessed using a plate reader.
Gene silencing and over-expression
siRNAs (including negative controls) were purchased from Dharmacon, RNAi Technologies. siRNAs (10 μg of annealed oligos) were transfected using Dharmacon transfection reagents, as recommended by the manufacturer. The cells were harvested 24–48 hours following transfection. For over-expression studies, we seeded the cells evenly in 6-well plates and, after overnight incubation, transfected 2 μg of each plasmid DNA using Fugene® Transfection Reagent (Promega Corporation).
Animals
C57BL/6J Apc Min/+ mice were crossbred with either C57BL/6J or C57BL/6J Pla2g7−/− mice (12) to generate animals that carried a mutant Apc allele and two or zero functional Pla2g7 alleles (Apc Min/+ and Apc Min/+/Pla2g7−/− respectively). The animals were observed daily for clinical signs and morbidity, and were sacrificed using CO2 asphyxiation when they reached approximately 100 days of age. We dissected and washed the colons as previously described (27), stained them using methylene blue, and then assessed the number of polyps and tumors using a dissecting microscope. Edelfosine studies were conducted on mice aged 34 days (range = 31–36 days) by supplementing the drinking water with 12.5 μg/ml edelfosine in 0.1% ethanol for an average of 65 days (range = 62–69 days). All experimentation was conducted following approval by the University of Utah’s Animal Care and Use Committee.
Results
Deletion of Pla2g7 decreases small intestinal polyp and colon tumor incidence in Apc Min/+ mice
To evaluate the impact of Pla2g7 on intestinal polyposis, we crossed Pla2g7−/− mice with Apc Min/+ animals; the characteristics of the cohorts are summarized in Table 1. The median age of the mice at the time of analysis was 98 days and the proportion of male and female animals was comparable in the two groups. We found that deletion of Pla2g7 was associated with decreased polyposis in the proximal, mid, and distal regions of the small intestine (Table 1). In addition, the number of colonic tumors was significantly lower in Apc Min/+/Pla2g7−/−compared with Apc Min/+ mice (Table 1). All differences remained statistically significant when female and male mice were analyzed separately. Within each genotype, gender did not affect the number of intestinal polyps.
Table 1.
Deletion of Pla2g7 decreases polyp number in the Apc Min/+ model of intestinal polyposis
| Apc Min/+ | Apc Min/+/Pla2g7−/− | p | |
|---|---|---|---|
| n | 17 | 21 | - |
| Number of males (%) | 7 (41%) | 12 (57%) | - |
| Number of females (%) | 10 (59%) | 9 (43%) | - |
| Median age, days | 98 | 98 | - |
| Age range, days | 84 – 100 | 78 – 102 | - |
| Proximal intestinal polyps * | 5.4 ± 2.6 | 3.0 ± 2.2 | 0.0010 |
| Mid intestinal polyps * | 26.1 ± 12.3 | 12.0 ± 7.6 | 5.2 × 10−5 |
| Distal intestinal polyps * | 42.0 ± 17.4 | 22.1 ± 16.0 | 0.0004 |
| Colon polyps * | 0.6 ± 1.7 | 0.3 ± 0.6 | 0.2458 |
| Colon tumors** | 1.6 ± 1.3 | 0.5 ± 0.9 | 0.0020 |
Data reported represent the average number of polyps per mouse ± S.D.
Data reported represent the average number of tumors per mouse ± S.D.
cPAF inhibits proliferation and induces apoptosis of colonic epithelial cells through the PAF receptor
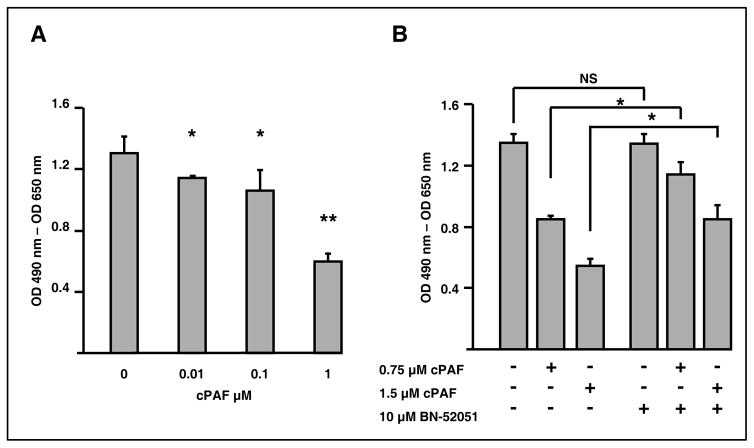
To elucidate the mechanism whereby absence of PLA2G7 decreased intestinal polyposis, we evaluated responses elicited following exposure of colon cancer cells to PAF. Previous studies showed that PAF mediates differentiation, proliferation, and growth inhibitory responses, depending on the biological context (4, 8). To characterize effects in the intestinal epithelium, we exposed HT-29 colonic epithelial cells to PAF (0–1 μM) in medium containing relatively low (0.5%) serum levels to limit PAF degradation by PLA2G7. PAF modestly inhibited cellular growth (not shown), but stronger growth inhibitory effects were observed with methylcarbamoyl PAF (cPAF), a metabolically stable analog that is resistant to hydrolysis by PLA2G7 (Fig. 1A). The concentrations used to assess effects on cellular growth were below the CMC of PAF [reported to be 2.5–3.0 μM, (28)], suggesting that the observed effects were the consequence of lipid signaling events and did not represent nonspecific cellular lysis. We next assessed whether participation of PAFR was required for growth inhibitory effects. Supplementation with the PAFR antagonist BN-52051 rescued cPAF-induced inhibition of cellular growth (Fig. 1B), establishing that in colonic epithelial cells cPAF inhibits cellular proliferation via PAFR.
Figure 1. Treatment of HT-29 cells with cPAF decreases proliferation of colon cancer cells.
A. HT-29 cells seeded in 96-well plates were allowed to grow for 48 hours in media containing 10% FBS. The growth medium then was removed and the cells were exposed to the indicated concentrations of cPAF in media containing 0.1% FBS. After 72 hours at 37°C, we assessed cellular proliferation using the XTT assay, as described in Materials and Methods. *, p<0.05; **, p<0.005.
B. HT-29 cells seeded in 96-well plates were allowed to grow for 24 hours in media containing 10% FBS. The growth medium then was removed and the cells were exposed to the indicated concentrations of cPAF and BN-52021 in serum-free medium. Fresh media containing cPAF and BN-52021 were supplied every 24 hours. After a total of 72 hours at 37°C, we assessed cellular proliferation using the XTT assay. *, p<0.05.
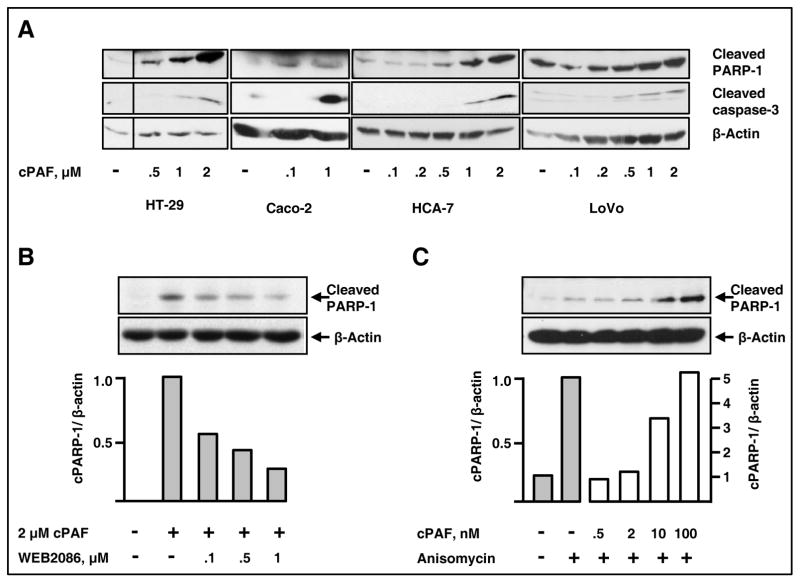
We next investigated the mechanisms accounting for cPAF-mediated effects on cellular survival. Lu and co-workers previously showed that in rat small intestinal epithelial cells PAF induces apoptosis by increasing caspase-3 activity and DNA fragmentation (29). Overnight treatment of serum-starved HT-29, Caco-2, HCA-7 and LoVo cells with cPAF increased caspase-3 activation and PARP-1 cleavage (Fig. 2A). While apoptosis in response to cPAF was observed in most colon cancer cells, we detected differences in the magnitude of individual responses; SW-480 cells were the most resistant (not shown). These variations may be due to differential susceptibility to PAF or altered function/expression of downstream effectors required for apoptosis in each cell line, or both. cPAF-induced apoptosis required PAFR expression as the specific PAFR antagonist WEB2086 inhibited this response (Fig. 2B) and lyso-PAF failed to induce PARP-1 cleavage (not shown). The pro-apoptotic agent anisomycin induced apoptosis, as expected, and low levels of cPAF potentiated this effect (Fig. 2C). These results suggest that cPAF can singly initiate the apoptotic cascade and also augment programmed cell death induced by other agents.
Figure 2. cPAF specifically induces apoptosis and promotes anisomycin-induced apoptosis in colon cancer cells.
A. HT-29, Caco-2, HCA-7 and LoVo cells were starved overnight in serum-free medium (HT-29 and Caco-2) or medium containing 0.1% serum (HCA-7 and LoVo). The next day, we subjected the cells to overnight (16 hours) treatment with vehicle, or the indicated concentrations of cPAF. Solubilized extracts then were subjected to electrophoresis and immunoblot analyses to assess caspase-3 activation and PARP-1 cleavage. β-actin levels were determined for normalization purposes.
B. Starved HT-29 cells were pre-incubated with the PAFR antagonist WEB2086 or with vehicle for 1 h at 37°C. We then added cPAF and incubated the cells overnight at 37°C. Protein extracts were subjected to electrophoresis and immunoblot analyses to assess PARP-1 cleavage and β-actin content. Band intensity was determined using GelQuant. NET, as described in Materials and Methods. cPARP-1 = cleaved PARP-1
C. HT-29 cells were starved for 48 h and then were pre-treated with vehicle or cPAF (0.5–100 nM) for 30 min before addition of anisomycin (25 μg/ml). The cells then were incubated for 6 h in the presence of anisomycin and cPAF. PARP-1 cleavage and β-actin content were determined as described above.
Basal and stimulated PAFR activation decrease pAkt473 through a G-protein-independent mechanism
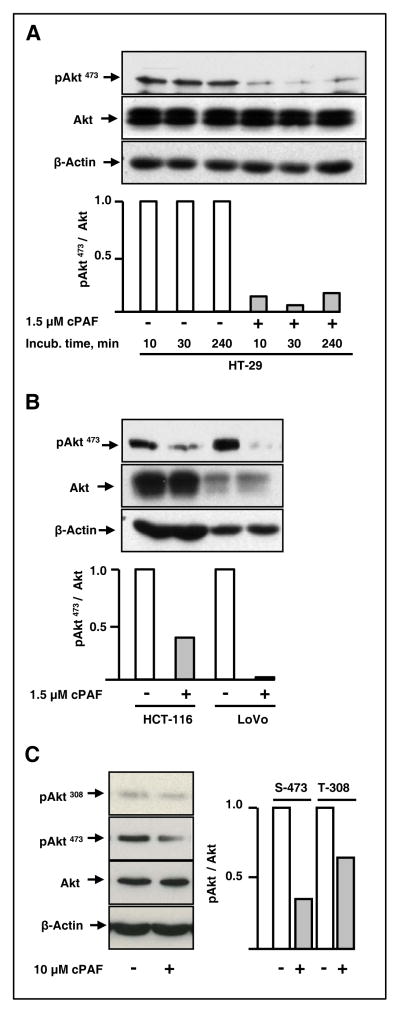
To identify downstream events following challenge with cPAF, we evaluated the state of activation of signaling pathways known to affect cellular growth, survival, and apoptosis. Akt has been implicated in the regulation of multiple biological processes ranging from glycogenesis to embryonic development, inflammation, apoptosis, and cell proliferation. Moreover, activation of the PI3K/Akt pathway plays a central role in tumorigenesis across a variety of malignancies. We found that cPAF consistently decreased the level of basal pAkt473 in HT-29 cells for relatively prolonged time periods (Fig. 3A). This response was not limited to a single colon cancer cell line because HCT-116, LoVo, and HCA-7 (not shown) cells responded in similar fashions (Fig. 3B). Slight variations in the state of basal cellular activation likely accounted for variability in the extent of pAkt473 de-phosphorylation among experiments. However, the response was consistently observed throughout the study (average decrease in pAkt473 = 65%, n=17). While cPAF affected the extent of Akt phosphorylation at serine-473, we observed modest effects on threonine-308 (64% versus 35% decrease in pAkt473 and pAkt308, respectively, Fig. 3C).
Figure 3. cPAF treatment decreases basal pAkt473 levels.
A. Starved HT-29 cells were treated with 1.5 μM cPAF or vehicle for 10, 30, and 240 min at 37°C. The levels of pAkt473, Akt, and β-actin then were determined in protein lysates, using immunoblot analyses.
B. Starved HCT-116 and LoVo cells were treated with 1.5 μM cPAF or vehicle for 30 min at 37°C. The levels of pAkt473, Akt, and β-actin then were determined using immunoblot analyses, as above.
C. Starved HT-29 cells were treated with cPAF or vehicle for 30 min at 37°C. The levels of pAkt308, pAkt473, Akt and β-actin then were determined as above.
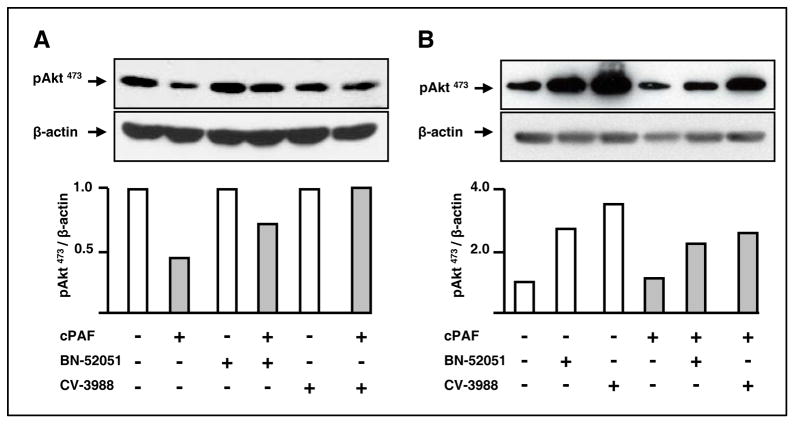
Consistent with our observations in cell growth assays, cPAF-mediated decrease in pAkt473 levels required PAFR, as the specific antagonists BN-52051 and CV-3988 partially prevented this effect (Fig. 4A). In addition, we detected increased levels of pAkt473 in HT-29 cells treated for 30 min with the same antagonists (Fig. 4B). These combined results indicate that in intestinal epithelial cells PAFR attenuates basal and stimulated Aktphosphorylation.
Figure 4. PAFR is required for cPAF-mediated de-phosphorylation of pAkt473.
A. HT-29 cells were starved overnight and then were incubated with 10 μM BN-52051 or CV-3988 for 3 h at 37°C. We then exposed the cells to 1.5 μM cPAF for 30 min and assessed the levels of pAkt473 and β-actin, as above.
B. HT-29 cells were starved overnight and then were incubated with 2 μM BN-52051 or CV-3988 for 30 min at 37°C. We then exposed the cells to 1.5 μM cPAF for 10 min and assessed the levels of pAkt473 and β-actin.
PAFR can interact with multiple G proteins and lead to activation of diverse signaling pathways (7). PAFR ligands have been shown to activate Gαi/Gα0, leading to decreased cAMP levels and protein kinase A (PKA) activity (7). Signaling through cAMP/PKA was previously reported to modulate the PI3K/Akt signaling axis in coronary microvessels (30). We tested the effect of pertussis toxin, which ribosylates Gαi/Gα0 in a Gαβγ heterotrimeric state-dependent fashion (7), and found that the inhibitor did not significantly affect cPAF-mediated de-phosphorylation of pAkt473 (Supplementary Fig. S1A). These results indicate that PAFR expressed on colonic epithelial cells does not couple to Gαi/Gα0. In addition, we found that inhibition and activation of PKA with KT-5720 and forskolin, respectively, did not affect cPAF-induced de-phosphorylation of pAkt473 in a manner consistent with a role of PKA in this response (Supplementary Fig. S1, panels B and C). Our studies suggest that PAFR negatively regulates pAkt473 levels through mechanisms that are largely independent of PKA and that do not require coupling to Gαi/Gα0.
In additional studies we tested whether Gαq and/or βγ subunits released from heterotrimeric G proteins were involved in de-phosphorylation of pAkt473. Functional coupling to Gαq activates PLC-β, increases production of inositol (1,4,5)-trisphosphate (6) and activates PI3K/Akt signaling. Our results (Fig. 3) showing that cPAF treatment decreases pAkt473 levels combined with the observation that PLC inhibitors had no effect on cPAF-induced effects on pAkt473 levels (not shown) suggest that PAFR coupling to Gαq is not required for reduced pAkt473. We next considered whether PKC activation, which usually occurs following coupling of PAFR to Gβγ subunits (7), was required for de-phosphorylation of pAkt473. We examined the effect of the PKC inhibitors bisindolylmaleimide-I (BIM-I, an inhibitor of classic and novel PKC subfamilies), Gö-6983 (a broad-spectrum PKC inhibitor), and Gö-6976 (an inhibitor of PKC-α and PKC-β). We found that these agents failed to block cPAF-mediated effects on pAkt473 levels (Supplementary Fig. S2, panels A and B). We conclude that the ability of cPAF to induce de-phosphorylation of pAkt473 does not require PAFR coupling to βγ subunits or PKC activation. Our combined observations suggest that mechanisms other than canonical signaling through G proteins are involved in cPAF-mediated de-phosphorylation of pAkt473. These results are somewhat reminiscent of studies showing that in certain cells, agonist stimulation of PAFR activates signaling pathways through G-protein-independent mechanisms (31).
The Akt phosphatase PHLPP2 and β-arrestin mediate cPAF-induced de-phosphorylation of pAkt473
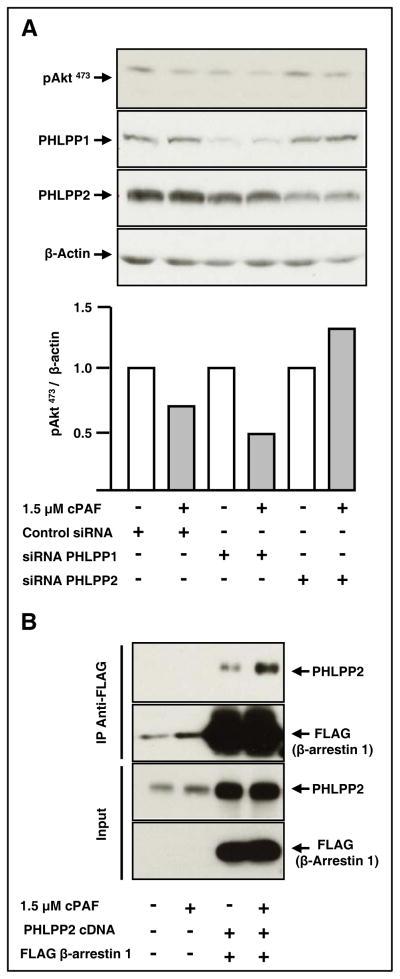
De-phosphorylation of pAkt473 is catalyzed by okadaic acid-sensitive protein phosphatases [PP, (32)] and by PHLPP enzymes [PH domain and leucine-rich repeat protein phosphatase, (33)]. We first tested whether cPAF treatment decreased phosphorylation of Aktthrough PP1 or PP2A. We found that calyculin A and okadaic acid, which inhibit both PP1 and PP2A (34), failed to antagonize cPAF-induced de-phosphorylation of pAkt473, suggesting lack of participation of these phosphatases (Supplementary Fig. S3, panels A and B). We next assessed whether PHLPP1 or PHLPP2, which dephosphorylate Serine-473 in Akt (33) were involved. We found that siRNA-mediated silencing of PHLPP2, but not that of PHLPP1, prevented cPAF-induced de-phosphorylation of pAkt473 (Fig. 5A).
Figure 5. The Akt phosphatase PHLPP2 forms a complex with β-arrestin 1 and mediates cPAF-induced de-phosphorylation of pAkt473.
A. HT-29 cells were transfected with control oligos, or with siRNAs for PHLPP1 or PHLPP2, as described in Materials and Methods. After 24 hours at 37°C, the cells were starved overnight in serum-free media. We then stimulated the cells with cPAF for 45 min at 37°C. Cellular proteins were harvested, and then subjected to electrophoresis and immunoblot analyses.
B. HT-29 cells were transfected with cDNAs encoding PHLPP2 and FLAG-tagged β-arrestin 1. After 24 h at 37°C, we starved the cells overnight, stimulated them with cPAF for 30 min at 37°C, and then incubated cell lysates with anti-FLAG beads overnight at 4°C. We assessed PHLPP2 and FLAG-β-arrestin 1 levels in total lysates to determine transfection efficiency. We determined the extent of cPAF-induced association between PHLPP2 and β-arrestin 1 by subjecting the proteins associated with FLAG beads to electrophoresis and immunoblotting, using antibodies against PHLPP2 and β-arrestin 1.
One of the mechanisms whereby receptor signaling events are blocked involves β-arrestin, a molecule long known to associate with activated receptors and limit further signaling (35). β-Arrestin recruitment to GPCRs is thought to terminate signaling by providing a scaffold for the assembly of complexes involving the PI3K, Ras, and ERK1/2 axes, among others (31, 36, 37). Previous studies have shown that PAFR agonists induce translocation of β-arrestins from the cytoplasm to the cell membrane and that PAFR binds β-arrestins (38). To investigate whether β-arrestins participate in cPAF/PHLPP2-induced de-phosphorylation of pAkt473, we expressed PHLPP2 and FLAG-tagged β-arrestin 1 in HT-29 cells, and then treated the cells with cPAF or vehicle. Immunoprecipitation of β-arrestin 1 revealed that cPAF specifically increased association between PHLPP2 and β-arrestin 1, and promoted de-phosphorylation of Akt at serine-473 (Fig. 5B), suggesting that cPAF decreases pAkt473 levels via β-arrestin 1 and PHLPP2.
Effects of Pla2g7 deletion and pharmacological activation of PAF/PAFR signaling in vivo
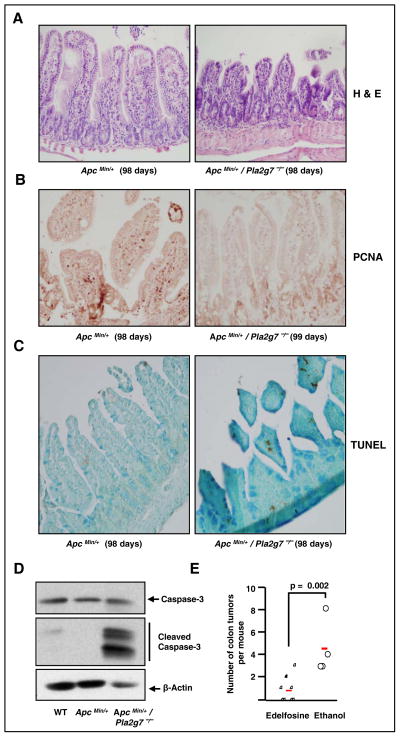
Our next goal was to assess whether functional changes resulting from stimulation with cPAF in cultured cells were recapitulated in vivo. First, we evaluated the consequences of deletion of Pla2g7 in Apc Min/+ mice, which is expected to result in substrate accumulation. We conducted histological and immunohistochemical analyses in distal small intestinal tissues from Apc Min/+ mice harboring 0 or 2 wild-type Pla2g7 alleles. H&E staining revealed similar intestinal architecture in Apc Min/+ intestinal tissues, regardless of Pla2g7 expression (Figure 6A). However, tissues from Apc Min/+ mice were characterized by the presence of several islands of proliferating cells that were absent in mice lacking expression of functional Pla2g7 alleles (Figure 6B). In addition, Apc Min/+/Pla2g7−/− mice expressed PCNA-positive epithelial cells primarily in the base region of the crypt, suggesting that deletion of Pla2g7 decreases epithelial cell proliferation along the villus axis. Staining for TUNEL demonstrated that intestinal apoptosis increased in the absence of Pla2g7 (Figure 6C). The positive effects of Pla2g7 deletion on apoptosis occur at relatively early stages of polyp development as Apc Min/+/Pla2g7−/− mice showed clear evidence of intestinal apoptosis at 5 weeks of life (Figure 6D). Finally, in vivo activation of PAF signaling by oral administration of edelfosine, a metabolically stable analog of PAF, significantly decreased the incidence of colon tumors in ApcMin/+ mice (Figure 6E).
Figure 6. Effect of Pla2g7 deletion and pharmacological activation of PAF/PAFR signaling in vivo.
A. H&E-stained sections from small intestine show well preserved villi in Apc Min/+ mice and atrophic-appearing villi in Apc Min/+/Pla2g7−/− mice. Original magnification = 20X.
B. Immunohistochemistry of PCNA shows higher labeling in epithelial cell nuclei from Apc Min/+ mice and scant labeling in Apc Min/+/Pla2g7−/− animals. Original magnification = 20X.
C. TUNEL assays show higher labeling in epithelial cells from Apc Min/+/Pla2g7−/− mice compared with Apc Min/+ mice. Original magnification = 20X.
D. Expression of caspase-3, cleaved caspase-3 and β-actin in distant intestinal tissue extracts from 5 week-old wild-type (WT), Apc Min/+, and Apc Min/+/Pla2g7−/− mice.
E. Effect of oral administration of edelfosine, a metabolically-stable PAF analog, on the incidence of colon tumors in Apc Min/+ mice.
PLA2G7 expression is elevated in colon tumors compared with normal tissues
Previous studies reported increased PLA2G7 activity (22, 23) and mRNA levels (24) in tumor tissues and plasma from colon cancer patients compared with samples from healthy donors. To investigate whether expression of PLA2G7 protein correlates with colon tumorigenesis in humans, we utilized publicly available information provided by The Swedish Human Protein Atlas project (39). These analyses revealed increased levels of PLA2G7 in colon adenocarcinomas compared with normal colonic tissues (Supplementary Table S1). This observation, combined with functional analyses in animal and cellular models [this study and ref. (24)] suggest that PLA2G7 actively contributes to the pathogenesis of colorectal cancer.
Discussion
Our results showed that in all regions of the small intestine analyzed, Apc Min/+ mice developed substantially less intestinal polyps and tumors in the absence of functional Pla2g7 alleles. Intestinal tissues from Pla2g7-null mice showed decreased proliferation when compared with tissues from control Apc Min/+ mice. These results were complemented with studies in cultured colonic epithelial cells exposed to a non-hydrolyzable analog of PAF. We found evidence for cPAF-mediated inhibition of cellular proliferation, caspase-3 activation and PARP-1 cleavage in a variety of colonic epithelial cells. These responses were mediated by PAFR and were associated with the formation of a complex between β-arrestin 1 and the Akt phosphatase PHLPP2. Taken together, our data suggest that cPAF-mediated de-phosphorylation of pAkt473 enhances apoptosis and attenuates activation of cancer-relevant downstream effector molecules, thus limiting proliferation and cell survival in vivo.
PLA2 activities play dual roles in carcinogenesis, and these functions are tissue- and context-dependent. These enzymes modulate the levels of biologically active lipids whose functions are strongly affected by the environment. For example, Pla2g2a encodes a secretory PLA2 (sPLA2-IIA) that reduces intestinal tumor development in ApcMin/+ mice (40) but enhances colon tumor cell growth and infiltration in a tumor explant model (41). Interestingly, the Pla2g2a gene is naturally disrupted in a number of mouse strains, including C57BL/6, the strain used in our studies (42). The physiological function of sPLA2-IIA likely differs from that of PLA2G7 as the enzymes metabolize different phospholipid substrates (43). Our studies in mice indicate that deletion of Pla2g7 attenuates intestinal polyposis, suggesting that the enzyme actively contributes to colon tumorigenesis. Consistently, PLA2G7 has been proposed to play a causal role in colon tumor formation downstream of oncogenic mutations (24). In addition, decreasing PLA2G7 expression in human prostate cancer cells limited migration and proliferation, and induced apoptosis (44), suggesting similar functions for PLA2G7 in prostate tumors.
Highly controlled, limited PAF production is likely to have multiple beneficial effects in the intestine. Normal mucosal epithelial cells are rapidly proliferating and completely turn over every 24 to 96 h and low-level activation of PAF signaling may contribute to the maintenance of a normal intestinal barrier, enhancing protection and facilitating repair from injury (45). Kelly et al. showed that bacterial translocation across the intestinal epithelial barrier requires PAFR and suggested that this may represent a physiological mechanism to clear bacteria (46). Our studies, combined with those of others (4), showed that activation of the PAF/PAFR axis may have beneficial effects in colon cancer by mediating growth-inhibitory, pro-apoptotic functions. Indeed, supplementation of the drinking water with edelfosine, a stable PAF analog, reduced the incidence of colon tumors in ApcMin/+ mice, in agreement with our studies in cultured cells.
It is well recognized that chronic bowel inflammation predisposes to colon cancer. Exacerbated signaling through the PAF axis is thought to negatively impact intestinal diseases such as necrotizing enterocolitis, ulcerative colitis and Crohn’s disease. Increased apoptosis can be highly detrimental in these settings which are characterized by exacerbated inflammation and chronic tissue damage. PAF is an early inducer of enterocyte apoptosis (47) during necrotizing enterocolitis and its likely accumulation resulting from deletion of Pla2g7 increases the severity of this disease (12). However, PAF levels appear to inversely correlate with the severity of colorectal cancer (22) and our data show that the pro-apoptotic effects of PAF limit intestinal polyposis. These combined observations strongly suggest that the role of PAF in intestinal diseases is multifaceted and highly dependent on its concentration, the nature of the disease, its stage, and biological context.
Our studies indicate that interaction of PAF with PAFR is required for induction of apoptosis, and that this response occurs in a G-protein-independent manner. PAFR ligand-induced recruitment of β-arrestins is independent of G protein activation (38) and requires PAFR phosphorylation (48). Interestingly, when arrestins recognize phosphorylated receptors they promote the formation of stable arrestin-receptor complexes (36) and the onset of more sustained signaling events compared with those resulting from G protein-mediated signaling. The relatively prolonged de-activation of pAkt473 observed in our study, combined with evidence suggesting participation of β-arrestin 1 in this response, lead us to speculate that cPAF induces phosphorylation of PAFR, β-arrestin recruitment, formation of stable β-arrestin-PAFR complexes, and sustained de-phosphorylation of pAkt473 through a G protein-independent pathway. Similarly, other GPCRs [i.e, dopamine 2 receptor (D2R) and protease-activated receptor-2] respond to ligand stimulation in this fashion (49).
Our findings that PAFR activation enhances de-phosphorylation of pAkt473 by PHLPP2, and that pAkt308 levels are modestly decreased are also novel. PHLPPs are phosphatases that specifically de-phosphorylate serine-473 in the hydrophobic phosphorylation motif. The expression of PHLPP1 and PHLPP2 is either lost or decreased in 78% and 86% of colon tumor tissues, respectively, suggesting that PHLPP is a colon cancer tumor suppressor (50). De-phosphorylation of Akt at both serine-473 and threonine-308 leads to a reduction of kinase activity and can result in activation of substrates such as glycogen synthase kinases 3α and 3β (GSK3α and GSK3β) that are negatively regulated by Akt. We found no evidence for cPAF-induced effects on GSK3β phosphorylation, which is consistent with modest cPAF-mediated effects on threonine-308. Instead, we found that cPAF-induced de-phosphorylation of pAkt473 inhibited the survival-promoting effects of Akt and induced apoptosis by activating caspase-3 and stimulating PARP-1 cleavage.
In summary, we have shown that constitutive deletion of Pla2g7 suppresses intestinal polyposis in the Apc Min/+ model. Our studies in mouse tissues and in intestinal epithelial cells suggest that this effect is the consequence of ligand-mediated activation of PAFR, decreased Akt activation through PHLPP2, and enhanced apoptosis. The study points at inhibition of PLA2G7 activity as a possible novel strategy for the treatment of inherited forms of colon cancer in which the function of APC is compromised. Our observations are potentially generalizable to sporadic forms of the disease, as mutations in APC are common to most colon cancers.
Supplementary Material
Acknowledgments
Grant Support
This work was supported by grant 5P01CA073992 from the National Cancer Institute and by the Huntsman Cancer Foundation. The work was also supported by access to technical cores supported by a Cancer Center Support Grant (P30CA042014) to the Huntsman Cancer Institute.
Footnotes
Conflicts of interest:none
References
- 1.Cheung AF, Carter AM, Kostova KK, Woodruff JF, Crowley D, Bronson RT, et al. Complete deletion of Apc results in severe polyposis in mice. Oncogene. 2011;29:1857–64. doi: 10.1038/onc.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–9. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Chakrabarty S. Platelet-activating factor activates mitogen-activated protein kinases, inhibits proliferation, induces differentiation and suppresses the malignant phenotype of human colon carcinoma cells. Oncogene. 2003;22:2186–91. doi: 10.1038/sj.onc.1206348. [DOI] [PubMed] [Google Scholar]
- 5.Kume K, Shimizu T. Platelet-activating factor (PAF) induces growth stimulation, inhibition, and suppression of oncogenic transformation in NRK cells overexpressing the PAF receptor. J Biol Chem. 1997;272:22898–904. doi: 10.1074/jbc.272.36.22898. [DOI] [PubMed] [Google Scholar]
- 6.Honda Z, Ishii S, Shimizu T. Platelet-activating factor receptor. J Biochem. 2002;131:773–9. doi: 10.1093/oxfordjournals.jbchem.a003164. [DOI] [PubMed] [Google Scholar]
- 7.Ishii S, Shimizu T. Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog Lipid Res. 2000;39:41–82. doi: 10.1016/s0163-7827(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 8.Aponte M, Jiang W, Lakkis M, Li MJ, Edwards D, Albitar L, et al. Activation of platelet-activating factor receptor and pleiotropic effects on tyrosine phospho-EGFR/Src/FAK/paxillin in ovarian cancer. Cancer Res. 2008;68:5839–48. doi: 10.1158/0008-5472.CAN-07-5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longo WE, Polities G, Vernava AM, 3rd, Deshpande Y, Niehoff M, Chandel B, et al. Platelet-activating factor mediates trinitrobenzene induced colitis. Prostaglandins Leukot Essent Fatty Acids. 1994;51:419–24. doi: 10.1016/0952-3278(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 10.Caplan MS, Kelly A, Hsueh W. Endotoxin and hypoxia-induced intestinal necrosis in rats: the role of platelet activating factor. Pediatr Res. 1992;31:428–34. doi: 10.1203/00006450-199205000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Wallace JL, Steel G, Whittle BJ, Lagente V, Vargaftig B. Evidence for platelet-activating factor as a mediator of endotoxin-induced gastrointestinal damage in the rat. Effects of three platelet-activating factor antagonists. Gastroenterology. 1987;93:765–73. doi: 10.1016/0016-5085(87)90438-0. [DOI] [PubMed] [Google Scholar]
- 12.Lu J, Pierce M, Franklin A, Jilling T, Stafforini DM, Caplan M. Dual roles of endogenous platelet-activating factor acetylhydrolase in a murine model of necrotizing enterocolitis. Pediatr Res. 2010;68:225–30. doi: 10.1203/PDR.0b013e3181eb2efe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braeuer RR, Zigler M, Villares GJ, Dobroff AS, Bar-Eli M. Transcriptional control of melanoma metastasis: the importance of the tumor microenvironment. Seminars in cancer biology. 2011;21:83–8. doi: 10.1016/j.semcancer.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao Y, Wolverton JE, Zhang Q, Marathe GK, Al-Hassani M, Konger RL, et al. Ultraviolet B radiation generated platelet-activating factor receptor agonist formation involves EGF-R-mediated reactive oxygen species. J Immunol. 2009;182:2842–8. doi: 10.4049/jimmunol.0802689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HA, Seo KH, Kang YR, Ko HM, Kim KJ, Back HK, et al. Mechanisms of platelet-activating factor-induced enhancement of VEGF expression. Cell Physiol Biochem. 2011;27:55–62. doi: 10.1159/000325205. [DOI] [PubMed] [Google Scholar]
- 16.Qu X, Huang L, Burthart T, Crawford SE, Caplan MS, Hsueh W. Endotoxin induces PAF production in the rat ileum: quantitation of tissue PAF by an improved method. Prostaglandins. 1996;51:249–62. doi: 10.1016/0090-6980(96)00020-2. [DOI] [PubMed] [Google Scholar]
- 17.Claud EC, Lu J, Wang XQ, Abe M, Petrof EO, Sun J, et al. Platelet-activating factor-induced chloride channel activation is associated with intracellular acidosis and apoptosis of intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1191–200. doi: 10.1152/ajpgi.00318.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan XD, Chang H, Qu XW, Caplan M, Gonzalez-Crussi F, Hsueh W. Platelet-activating factor increases mucosal permeability in rat intestine via tyrosine phosphorylation of E-cadherin. Br J Pharmacol. 2000;129:1522–9. doi: 10.1038/sj.bjp.0702939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker MA, Bazan HE, Marcheselli V, Rodriguez de Turco EB, Bazan NG. Platelet-activating factor induces permeability transition and cytochrome c release in isolated brain mitochondria. J Neurosci Res. 2002;69:39–50. doi: 10.1002/jnr.10235. [DOI] [PubMed] [Google Scholar]
- 20.Wu B, Iwakiri R, Ootani A, Fujise T, Tsunada S, Fujimoto K. Platelet-activating factor promotes mucosal apoptosis via FasL-mediating caspase-9 active pathway in rat small intestine after ischemia-reperfusion. Faseb J. 2003;17:1156–8. doi: 10.1096/fj.02-0499fje. [DOI] [PubMed] [Google Scholar]
- 21.Denizot Y, Gainant A, Guglielmi L, Bouvier S, Cubertafond P, Mathonnet M. Tissue concentrations of platelet-activating factor in colorectal carcinoma: inverse relationships with Dukes’ stage of patients. Oncogene. 2003;22:7222–4. doi: 10.1038/sj.onc.1207032. [DOI] [PubMed] [Google Scholar]
- 22.Denizot Y, Truffinet V, Bouvier S, Gainant A, Cubertafond P, Mathonnet M. Elevated plasma phospholipase A2 and platelet-activating factor acetylhydrolase activity in colorectal cancer. Mediators Inflamm. 2004;13(1):53–4. doi: 10.1080/09629350410001664824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denizot Y, Descottes B, Truffinet V, Valleix D, Labrousse F, Mathonnet M. Platelet-activating factor and liver metastasis of colorectal cancer. Int J Cancer. 2005;113:503–5. doi: 10.1002/ijc.20585. [DOI] [PubMed] [Google Scholar]
- 24.McMurray HR, Sampson ER, Compitello G, Kinsey C, Newman L, Smith B, et al. Synergistic response to oncogenic mutations defines gene class critical to cancer phenotype. Nature. 2008;453:1112–6. doi: 10.1038/nature06973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taketo MM. Mouse models of gastrointestinal tumors. Cancer Sci. 2006;97:355–61. doi: 10.1111/j.1349-7006.2006.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–70. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 27.Al-Salihi MA, Terrece Pearman A, Doan T, Reichert EC, Rosenberg DW, Prescott SM, et al. Transgenic expression of cyclooxygenase-2 in mouse intestine epithelium is insufficient to initiate tumorigenesis but promotes tumor progression. Cancer Lett. 2009;273:225–32. doi: 10.1016/j.canlet.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blank ML, Lee T, Fitzgerald V, Snyder F. A specific acetylhydrolase for 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (a hypotensive and platelet-activating lipid) J Biol Chem. 1981;256:175–8. [PubMed] [Google Scholar]
- 29.Lu J, Caplan MS, Li D, Jilling T. Polyunsaturated fatty acids block platelet-activating factor-induced phosphatidylinositol 3 kinase/Akt-mediated apoptosis in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1181–90. doi: 10.1152/ajpgi.00343.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang XP, Hintze TH. cAMP signal transduction induces eNOS activation by promoting PKB phosphorylation. Am J Physiol Heart Circ Physiol. 2006;290:H2376–84. doi: 10.1152/ajpheart.00614.2005. [DOI] [PubMed] [Google Scholar]
- 31.Chen Z, Rola-Pleszczynski M, Stankova J. Activation of ERK1/2 by platelet-activating factor receptor is independent of receptor internalisation and G-protein activation. Cell Signal. 2003;15:843–50. doi: 10.1016/s0898-6568(03)00056-1. [DOI] [PubMed] [Google Scholar]
- 32.Rocher G, Letourneux C, Lenormand P, Porteu F. Inhibition of B56-containing protein phosphatase 2As by the early response gene IEX-1 leads to control of Akt activity. J Biol Chem. 2007;282:5468–77. doi: 10.1074/jbc.M609712200. [DOI] [PubMed] [Google Scholar]
- 33.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 34.McCluskey A, Sakoff JA. Small molecule inhibitors of serine/threonine protein phosphatases. Mini Rev Med Chem. 2001;1:43–55. doi: 10.2174/1389557013407205. [DOI] [PubMed] [Google Scholar]
- 35.Grady EF. Cell signaling. Beta-arrestin, a two-fisted terminator. Science. 2007;315:605–6. doi: 10.1126/science.1138505. [DOI] [PubMed] [Google Scholar]
- 36.DeFea KA. Beta-arrestins as regulators of signal termination and transduction: how do they determine what to scaffold? Cell Signal. 23:621–9. doi: 10.1016/j.cellsig.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–7. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 38.Chen Z, Dupre DJ, Le Gouill C, Rola-Pleszczynski M, Stankova J. Agonist-induced internalization of the platelet-activating factor receptor is dependent on arrestins but independent of G-protein activation. Role of the C terminus and the (D/N)PXXY motif. J Biol Chem. 2002;277:7356–62. doi: 10.1074/jbc.M110058200. [DOI] [PubMed] [Google Scholar]
- 39.Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, et al. Towards a knowledge-based Human Protein Atlas. Nature biotechnology. 2010;28:1248–50. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 40.Cormier RT, Hong KH, Halberg RB, Hawkins TL, Richardson P, Mulherkar R, et al. Secretory phospholipase Pla2g2a confers resistance to intestinal tumorigenesis. Nature genetics. 1997;17:88–91. doi: 10.1038/ng0997-88. [DOI] [PubMed] [Google Scholar]
- 41.Belinsky GS, Rajan TV, Saria EA, Giardina C, Rosenberg DW. Expression of secretory phospholipase A2 in colon tumor cells potentiates tumor growth. Molecular carcinogenesis. 2007;46:106–16. doi: 10.1002/mc.20271. [DOI] [PubMed] [Google Scholar]
- 42.Kennedy BP, Payette P, Mudgett J, Vadas P, Pruzanski W, Kwan M, et al. A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains. J Biol Chem. 1995;270:22378–85. doi: 10.1074/jbc.270.38.22378. [DOI] [PubMed] [Google Scholar]
- 43.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50 (Suppl):S237–42. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vainio P, Lehtinen L, Mirtti T, Hilvo M, Seppanen-Laakso T, Virtanen J, et al. Phospholipase PLA2G7, associated with aggressive prostate cancer, promotes prostate cancer cell migration and invasion and is inhibited by statins. Oncotarget. 2011;2:1176–90. doi: 10.18632/oncotarget.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Keely S, Glover LE, Weissmueller T, MacManus CF, Fillon S, Fennimore B, et al. Hypoxia-inducible factor-dependent regulation of platelet-activating factor receptor as a route for gram-positive bacterial translocation across epithelia. Mol Biol Cell. 2010;21:538–46. doi: 10.1091/mbc.E09-07-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jilling T, Lu J, Jackson M, Caplan MS. Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental rat model of neonatal necrotizing enterocolitis. Pediatr Res. 2004;55:622–9. doi: 10.1203/01.PDR.0000113463.70435.74. [DOI] [PubMed] [Google Scholar]
- 48.Ahamed J, Ali H. Distinct roles of receptor phosphorylation, G protein usage, and mitogen-activated protein kinase activation on platelet activating factor-induced leukotriene C(4) generation and chemokine production. J Biol Chem. 2002;277:22685–91. doi: 10.1074/jbc.M110210200. [DOI] [PubMed] [Google Scholar]
- 49.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–73. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 50.Liu J, Weiss HL, Rychahou P, Jackson LN, Evers BM, Gao T. Loss of PHLPP expression in colon cancer: role in proliferation and tumorigenesis. Oncogene. 2009;28:994–1004. doi: 10.1038/onc.2008.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.