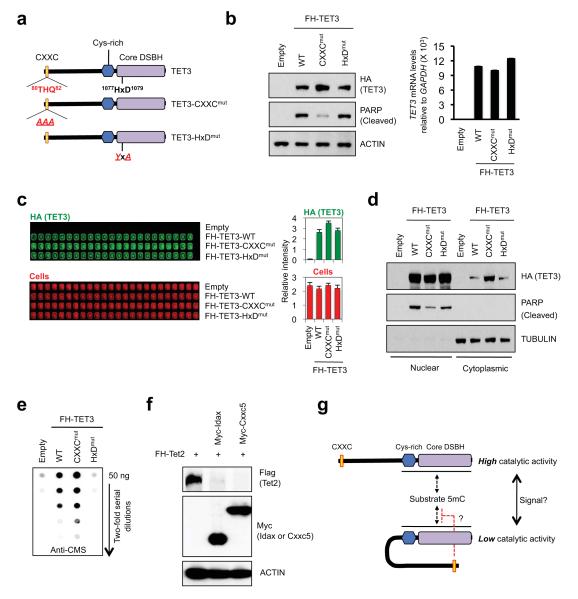
Fig. 4. Negative regulation of TET3 by its CXXC domain.
a. Schematic representation of TET3, TET3-CXXCmut and catalytically-inactive TET3-HxDmut.
b. Increased protein expression of TET3-CXXCmut relative to WT TET3 or TET3-HxDmut (left panel), without a change in TET3 mRNA levels (right panel).
c. In-cell western assays confirm that TET3-CXXCmut is expressed at higher levels than WT TET3 or TET3-HxDmut.
d. WT TET3 and TET3-HxDmut are mainly nuclear, whereas TET3-CXXCmut is found in both cytoplasmic and nuclear fractions and is less effective at inducing PARP cleavage than WT TET3.
e. Cells expressing TET3-CXXCmut show higher genomic 5hmC than cells expressing WT TET3 or TET3-HxDmut (anti-CMS dot blot3).
f. Cxxc5 expression results in a decrease in protein levels of co-expressed Tet2 in HEK293T cells.
g. Potential intramolecular (auto-inhibitory) interaction between the linked CXXC and catalytic domains of TET3.