Abstract
Osteoblasts (OBs) and adipocytes (APs) share a common mesenchymal ancestor. It is now clear that mesenchymal stem cell (MSC) maturation along the OB lineage comes at the expense of adipogenesis and vice versa. During aging, this balance increasingly favors the formation of APs. Hematopoiesis also slowly declines during the aging process. The role of OB lineage cells in hematopoiesis has been studied, but less is known about how APs regulate hematopoiesis. A few studies have demonstrated a negative relationship between APs and hematopoiesis; however, there is also evidence that brown adipose tissue (BAT) may promote hematopoiesis. This review will examine the current knowledge of how adipogenesis and osteogenesis change with aging and the implications of this changing environment on hematopoeisis.
Keywords: Mesenchymal stem cells, adipocyte, osteoblast, aging, osteoporosis, hematopoiesis
Introduction
It is an unfortunate fact of life that as humans age, they have a tendency to increase the proportion of their body weight made up by adipose tissue. In fact, it has been estimated that between the ages of 20 and 50, total body fat content doubles in males and females [1]. While body fat is increasing, bone mineral density, which peaks in the third decade of life, gradually declines [2]. Concomitant with these changes, there is also a decline in hematopoiesis with aging affecting both myeloid and lymphoid lineage cells accompanied by a gradual replacement of bone marrow stromal cells with adipose tissue [3, 4]. As osteoblasts (OBs) and adipocytes (APs) share a common mesenchymal ancestor [5] and are in intimate contact with hematopoietic cells within the bone marrow, understanding how aging affects the development and interactions between these cell lineages may ultimately help older individuals combat bone loss diseases and hematopoietic disorders.
In this review, we will examine the current understanding of how bone and adipose tissue formation changes with aging. Since bone and adipose tissue are two main components of the hematopoietic niche, we will also examine the impact of age-related differentiation changes in mesenchymal stem cells (MSCs) on the functional capacity of the hematopoietic system.
Mesenchymal Stem Cell Differentiation
MSCs have the potential to differentiate into several cell types including OBs, APs, chondrocytes, tenocytes, and myocytes [6]. For OB development, activation of runt-related transcription factor 2 (Runx2) is an initiating event in the commitment of the MSC to the osteo/chondroprogenitor line [7]. Further downstream, members of the Smad family of proteins, β-catenin and Osterix are also involved in the formation of terminally differentiated OBs [8]. On the other hand, CCAAT enhancer binding protein alpha (C/EBPα) and peroxisome proliferatoractivated receptor gamma (PPARγ) are the key transcription factors involved in AP formation [9].
Other important factors in MSC differentiation include the canonical Wnt pathway (henceforth referred to as Wnt), which signals through elevations in intracellular levels of β- catenin. Wnt plays a critical role in MSC differentiation down the OB lineage by increasing expression of osteogenic genes, such as Runx2, in developing OBs [10]. In AP development, Wnt acts to increase the mitotic activity of preadipocytes but has a negative effect on their maturation through inhibition of C/EBPα and PPARγ [11, 12]. The Wnt pathway may also suppress adipogenesis via the actions of glycogen synthase kinase-3β (GSK-3β). In the absence of Wnt signaling, GSK-3β phosphorylates β-catenin and targets it for ubiquination, therefore increases in Wnt signaling inhibit this GSK-3β-mediated destruction of β-catenin allowing the accumulation of β-catenin within the cell [13]. We will now examine how these factors change with aging, and the influence these changes have on the balance between osteoblastogenesis and adipogenesis.
Osteoblastogenesis in Aging
Osteoporosis affects approximately 2.8 million men and 9.1 million women in the United States, and osteopenia, or low bone mass, is estimated to affect an additional 14.4 million men and 26 million women [14]. These staggering numbers have stimulated much research into the causes and treatment of bone loss[14]. The current understanding of the pathogenesis of age-related bone loss implicates decreased efficiency of the basic multicellular unit, in which the OB deposits less new bone than the osteoclast resorbs [15]. Over time, this imbalance in bone metabolism leads to trabecular thinning, porosity, and fragile bones that are more likely to fracture [15]. How does the aging process contribute to this dysfunction in OBs and/or their mesenchymal precursors?
One of the mechanisms of OB activity decline appears to be a decrease in the pool and lifespan of MSCs from which they develop. It has been difficult to study human MSC numbers by modalities such as flow cytometry as there is a lack of a well-defined subset of markers that can completely encompass the population of MSCs [16]. However, several studies have used indirect methods to quantitatively assess the number of MSCs in young and older individuals. In 1999 D’Ippolito et al cultured MSCs isolated from males and females aged 3–70 years. This group found that MSCs isolated from “older” donors (aged 41 and above) had about an 80 percent reduction in the number of alkaline phosphatase positive colonies formed in vitro, thus demonstrating the decreased osteogenic potential of aging MSCs [17]. More recently, Zhou et al further examined the intrinsic effects of aging on human MSCs in vitro. Using bone marrow from human patients aged 17–90 as a source of MSCs, the group found that MSCs from older patients were more apoptotic, had higher expression of p53, p53 pathway genes p21 and BAX, and also generated fewer OBs than their younger counterparts [18]. The relative importance of MSC number alone in age-related bone loss is unclear as studies have determined that the greatest loss of MSCs occurs after reaching skeletal maturity with little decline thereafter [19, 20].
In addition to the decline in the pool of precursor cells from which OBs can develop, there are also declines in the lifespan of these precursors in aging. Stenderup et al conducted long term cultures of MSCs derived from young and elderly donors (age 19–29 years, and 68–81 years, respectively) and found that MSCs from older donors had significantly decreased numbers of population doublings (24 vs 41 , p<0.05, [21]). Perhaps it is the combination of a smaller MSC pool and decreased longevity that contribute to the decreased bone formation in aging.
There are also age-related changes that directly affect OB differentiation and function. Rauner et al recently studied the expression of Wnt-related proteins in young, adult, and old C57BL/6 mice (6 weeks, 6 months, and 18 months of age, respectively). In human chronology, young mice represent humans who have not reached skeletal maturity, adult mice represent skeletally mature adults between the ages of 30 and 50 and old mice represent humans older than 50. In bone tissue from old mice, there was an overall decreased expression of Wnt related proteins and OB differentiation was reduced [22]. Interestingly, reactive oxygen species (ROS), substances which are known to damage cells and which are cleared less efficiently with age have been found to interfere with β-catenin driven Wnt signaling [23, 24]. Specifically, ROS activate Forkhead box O (FoxO) transcription factors which in turn increase the expression of free radical scavengers. This induces increased association of FoxO with β- catenin, thus reducing the availability of β-catenin to activate transcription factors involved in bone formation [23, 24].
Dysfunctional genome maintenance also plays a role in the decline of aging MSCs. Several authors have shown that lower levels of the enzymes telomerase (protects DNA from shortening after replication) and helicase (aids DNA repair) seen in older animals are associated with decreased bone mass [25–28]. In an in vitro model, a human mesenchymal cell line stably transduced with a gene for a subunit of the human telomerase gene maintained the ability to differentiate down the OB lineage over hundreds of population doublings, also forming more bone when implanted into immunodeficient mice [29]. Using murine models of accelerated aging including mice deficient in telomerase, Werner helicase, or both, Wang et al determined that shortened telomeres in MSCs led to decreased expression of Runx2, increased expression of p53 and p21, and the formation of fewer mature OBs [30].
The study of a particular human genetic disease, called Hutchinson-Gilford Progeria Syndrome, has also contributed new insights into how bone and fat are maintained in old age. Children affected with this syndrome have a mutation in the LMNA gene, which encodes a nuclear envelope protein, lamin A [31]. As a result of this mutation, processing of lamin A is disturbed and the nuclei within the cells of these patients have altered shapes and strength, which disrupts mitosis and ultimately alters gene expression [32, 33]. The phenotypical manifestations of this disease include skeletal hypoplasia, short stature, pathologic fractures, hypertension, hyperlipidemia, arterial stenosis, lipodystrophy and shortened lifespan [33]. The Zmpste24-null mouse model of progeria lacks the enzyme to convert prelamin A to lamin A and therefore prelamin A accumulates within the nuclei of all cells [32]. In 2009, Rivas et al demonstrated that compared to age-matched wild-type mice, Zmpste24-null mice had a pattern of advanced osteopenia with increased levels of bone marrow fat [32]. These mice also had severely depressed levels of Runx2 expression and subsequently significantly fewer OBs and osteocytes while at the same time, expression of the adipogenic genes PPARγ and C/EBPα were elevated [32]. Similarly, Akter et al showed that knocking down lamin A expression in human MSCs, while it did not decrease Runx2 expression, did affect its nuclear binding ability, thus decreasing osteoblastogenesis and promoting adipogenesis [34]. These data implicate the involvement of lamin A, or deficiencies in the processing thereof, in age-related bone loss. Overall, it appears that in aging, there are multiple factors that decrease Runx2 activity, thus favoring PPARγ and driving the fate of MSCs towards adipogenesis.
Mature APs can also inhibit osteogenic function; therefore, the increased numbers of APs in aging may directly inhibit bone formation. In recent in vitro experiments, Lui et al showed that primary fat cells or fully differentiated 3T3-L1 cells (a pre-adipocyte cell line [35, 36]) co-cultured with partially differentiated OBs decreased Runx2 expression and alkaline phosphatase activity. These changes were attributed to substances released by APs that activate PPARγ within the OB, as silencing of PPARγ in the OB cell line rescued Runx2 expression [35]. There were also increases in adiponectin, which is a cytokine highly expressed by APs . In these experiments, adiponectin alone had no effect on Runx2, osteocalcin or alkaline phosphatase expression in OBs [35]. These results suggest that PPARγ influences the balance between OBs and APs even among more differentiated cells.
In vitro, exposure of MSCs to 1,25-dihydroxyvitamin D3 (1,25-VitD), the active form of vitamin D, increases their differentiation to OBs [37]. 25-hydroxyvitamin D3 (25-VitD), the inactive form of vitamin D, also has this effect on MSCs [38]. This discovery led to the finding that MSCs manufacture the 1α-hydroxylase CYP27B1, which can activate 25-VitD into the active form [38]. Interestingly, when exposed to 25-VitD, MSCs from older humans (age greater than 55) have a decreased ability to produce CYP27B1 [39]. Geng et al showed that in vitro, this decline can be rescued by administration of parathyroid hormone (PTH) [39]. PTH was able to upregulate the expression and activity of CYP27B1 in MSCs. This effect explains, at least in part, the efficacy of PTH in osteoporosis treatment. We will revisit the role of 1,25-VitD in MSC differentiation later, as it also appears to play a role in inhibiting adipogenesis.
Adipogenesis in Aging
Unlike osteoblastogenesis, which declines with age, adipogenesis seems to accelerate with aging. This paradigm was first demonstrated in the 1990’s in the senescence accelerated mice-P6 (SAMP6). The SAMP6 mouse begins developing osteoporosis within a few months of its birth [40]. Three years after the introduction of the SAMP6 mouse strain, Kajkenova et al, determined that SAMP6 mice not only had decreased osteoblastogenesis, but also increased numbers of mature APs within their bone marrow [41]. This finding of increased bone marrow fat with aging was soon translated to humans. In 2001, Justesen et al compared iliac crest bone biopsies of healthy individuals with osteoporotic individuals: compared to the total volume of the specimen, they found an age-related decrease in bone volume (BV/TV) concurrently with an increase in adipose tissue volume [4]. As the evidence mounted of an inverse relationship between osteoblastogenesis and adipogenesis, the search was on for the underlying mechanisms behind this shift in the balance.
With the increases in fat deposition with aging, it would be expected that the expression of adipogenic transcription factors would also increase. In 2004, Moerman et al demonstrated exactly this in mice. Specifically, this study showed that PPARγ expression was elevated in 26 week-old mice compared to 8 week-old mice. Furthermore, MSCs obtained from the bone marrow of the older mice were better able to spontaneously differentiate into APs in vitro, without requiring any stimulus [42]. What is it about aging that activates the adipogenic pathway in MSCs? The answer may lie with the increased oxidative stress that accompanies aging. As discussed above, these stresses reduce osteoblastogenesis in favor of adipogenesis.
As previously discussed, mammalian cells are under constant attack by free radicals, and the ability of our bodies to neutralize these threats decreases with aging [43]. Interestingly, besides the indirect effects on osteogenesis through binding β-catenin, evidence is emerging that these free radicals may directly induce PPARγ expression and stimulate adipogenesis. Recently, Almeida et al found that levels of oxidized lipids, which can act as free radicals, were elevated in older vs. younger mice. Furthermore, these oxidized lipids increased PPARγ expression and promoted apoptosis of OB lineage cells [44]. In addition, heme oxygenase-1 (HO-1), an agent known to neutralize oxidative stress [45], has been found to influence MSC differentiation. Suppression of HO-1 was found to strongly increase PPARγ expression and adipogenesis in human MSCs [46]. Taken together, it seems that increasing intracellular oxidative stress may be one of the major drivers of the switch from osteogenesis to adipogenesis during the aging process. Other factors, although contributing less, may also be regulating the balance between fat and bone in aging.
Even in healthy elderly individuals, low levels of vitamin D are commonly seen [47]. As discussed previously, vitamin D plays a role in stimulating OB differentiation. Moreover, vitamin D has been shown to directly inhibit adipogenesis in vitro using wild-type murine bone marrow stromal cells [48]. The mechanism of this inhibition was found to be suppression of PPARγ type 2 expression in the SAMP6 mouse model [49]. These data suggest that in the elderly population, vitamin D deficiency may mediate a synergistically negative effect on bone health by failing to stimulate osteoblastogenesis and enhancing adipogenesis. Also of importance when discussing osteoporosis, and perhaps the most widely thought of cause of osteoporosis in aging, is reduced estrogen levels (postmenopausal osteoporosis). Estrogen has also been implicated in increased bone marrow adiposity with aging.
In an abstract presented at the 2012 annual meeting of the American Society of Bone and Mineral Research, Krum and Wend found that estrogen receptor alpha knockout mice (ERαKO) had increased APs in the bone marrow compared to wild-type [50]. Ovariectomy was also able to induce this effect. Further analysis showed that the ERαKO mice had smaller, but more numerous lipid droplets within AP and that these mice also had higher levels of perilipin, an enzyme that promotes adipogenesis. These results suggest that estrogen may help to minimize AP accumulation in the bone marrow, an effect that would be diminished in the postmenopausal female. While it is clear that all of the above factors are converging to increase adipose tissue within the marrow cavity with age, the type of fat that is forming is also critical.
Not all fat is created equally. There are deposits of white and brown adipose tissue in the human, and each has different metabolic activities and functions. White adipose tissue (WAT) mainly stores energy in the form of triglycerides and cholesterol, with the individual cells containing few mitochondria. Conversely, brown adipose tissue (BAT) is composed of cells containing many mitochondria (hence the brown color), and act to dissipate energy in the form of heat [51]. Krings et al studied murine bone marrow adipose tissue in normal and diabetic mice, and determined that fat cells within the bone marrow possess characteristics of both white and brown adipose tissue. Specifically, marrow fat expressed gene markers associated with WAT, such as adiponectin and leptin, as well as markers for thermogenic proteins associated with BAT, such as deiodinase 2 and PPARγ coactivator 1α [52]. In this same study, the investigators also showed that with aging and diabetes, the expression of BAT-type gene markers in bone marrow decreased [52]. There has not been any specific link between BAT and osteogenesis, however, as discussed above, WAT may have direct inhibitory effects on OBs. Furthermore, there is some evidence that this progression of bone marrow fat away from the BAT phenotype may have implications for hematopoiesis as well.
Hematopoiesis in Aging
As the bone marrow also houses hematopoietic stem cells (HSCs), how does the aging bone marrow microenvironment affect the hematopoietic system? The deleterious effects of aging causes increases in anemia and weakened adaptive immune responses [53–57]. As both the myeloid and lymphoid lineages are affected, this suggests that HSCs, like MSCs, are also negatively affected by aging [58]. With HSCs, MSCs, OBs and adipose tissue all in close proximity within the bone marrow, how might their interactions influence hematopoiesis in old age?
In one of the earlier studies of interactions between hematopoietic lineage cells and APs, Belaid-Choucair et al showed that fibroblast like fat cells (FLFCs), which share many primary characteristics to unilocular fat cells, when co-cultured with monocyte and granulocyte precursors, were able to inhibit granulopoiesis [59]. They found that this inhibition was secondary to FLFC suppression of granulocyte-monocyte colony stimulating factor (GM-CSF) secretion by monocytes [59]. Addition of anti-NP1 antibody into these co-cultures abrogated this effect. NP1 is one of the receptors for vascular endothelial growth factor, is known to be expressed in WAT and bone marrow (BM) APs, and is known to suppress granulopoiesis [5, 60, 61].
Data generated from several other studies also support the hypothesis that APs do influence hematopoiesis. A 2009 study by Naveiras demonstrated several findings that implicate APs as negative regulators of hematopoiesis: in bone marrow regions of mice where APs are predominant, there were decreased numbers of HSCs and short-term repopulating cells; furthermore, transplantation of wild-type HSC into “fatless” mice (which cannot form APs) or treatment of transplanted wild-type mice with the PPARγ inhibitor bisphenol A diglycidyl ether (BADGE), resulted in enhanced repopulation of the hematopoietic system within the recipient mice [62]. More recently, Poncin et al studied the effects of the OB/AP balance within the marrow environment on hematopoietic recovery after irradiation in mice. These investigators found that while Runx2 expression peaked in MSCs one day after radiation treatment, PPARγ expression remained unchanged and then decreased significantly by day 7 [61]. The changes in Runx2 and PPARγ expression corresponded to hematopoietic precursor cell numbers, which reached a nadir by day 3 and were beginning to rebound by day 7 [61].
Along the same line, in 2012, Zhu et al examined hematopoietic recovery in mice following chemotherapy. Treatment of C57BL/6J mice with the PPARγ inhibitor BADGE after chemotherapy resulted in faster recovery of leukocytes, greater numbers of colony forming units in culture and increased numbers of HSCs within the bone marrow of these animals [63]. Taken together, these results suggest that adipogenesis is suppressed during times of increased hematopoietic demand.
Our laboratories recently studied the effects of APs on murine HSC maintenance. Here we cultured Lin-Sca+c-Kit+ cells for seven days (LSK cells, commonly regarded as HSCs [64]) with a mesenchymal stromal cell line that contained either low numbers of APs (GZL) or one that contained high numbers of APs (GZL/Adi) [65]. Co-culture of LSK cells with the GZL/Adi cells resulted in significantly fewer viable LSK cells after 7 days than co-culture with the GZL line [65]. There was also a significant elevation of NP1 expression in the GZL/Adi line compared to the GZL line [65]. Our findings are consistent with other studies that have found a negative relationship between APs and hematopoeisis, perhaps mediated by NP1. While these studies clearly implicate APs as negative regulators of hematopoiesis, other studies, involving BAT show an instance in which adipose tissue may promote hematopoiesis.
It is well-established that the bone marrow gradually fills with fat over time in humans. It is also known that humans rapidly lose deposits of BAT after birth, but it never completely disappears. As previously discussed, BAT is present within the bone marrow, and it too, appears to be lost in aging. Interestingly, very recent data are emerging that BAT is connected to hematopoiesis, although the mechanisms are incompletely understood. In 2012, using immunodeficient NOG mice, Nishio et al showed better engraftment of HSCs that had been cultured with human embryonic stem cell derived BAT (hESCdBA) than HSCs alone [66]. This hESCdBA was also found to express the hematopoietic cytokines thrombopoietin, interleukin-6, interleukin-3, colony-stimulating factors 3 and 2, as well as erythropoietin [66]. These studies highlight the complex interactions occurring in the BM microenvironment and how much work needs to be done to fully elucidate the mechanisms by which adipose tissue may be regulating hematopoiesis.
While the connections between adipose tissue and hematopoeisis are developing, the involvement of OBs in the HSC niche and hematopoeisis is much better understood [67–69]. In turn, several investigators recently reported that HSCs participate in OB differentiation [70, 71]. This reciprocal relationship may play a role in the decline of both hematopoiesis and osteogenesis with aging. Our laboratory recently demonstrated that the maturational status of OBs is important in their ability to stimulate HSCs. Specifically, we showed that OBs with higher Runx2 expression and low osteocalcin expression (i.e., less mature OBs) co-cultured with LSK cells significantly enhanced the in vitro generation of phenotypically defined progenitor cells and colony forming units (CFUs) compared to co-culture with more mature OBs [72]. As previously discussed, MSC differentiation in aging favors the AP lineage, therefore leaving fewer early OBs to support hematopoiesis.
Conclusions
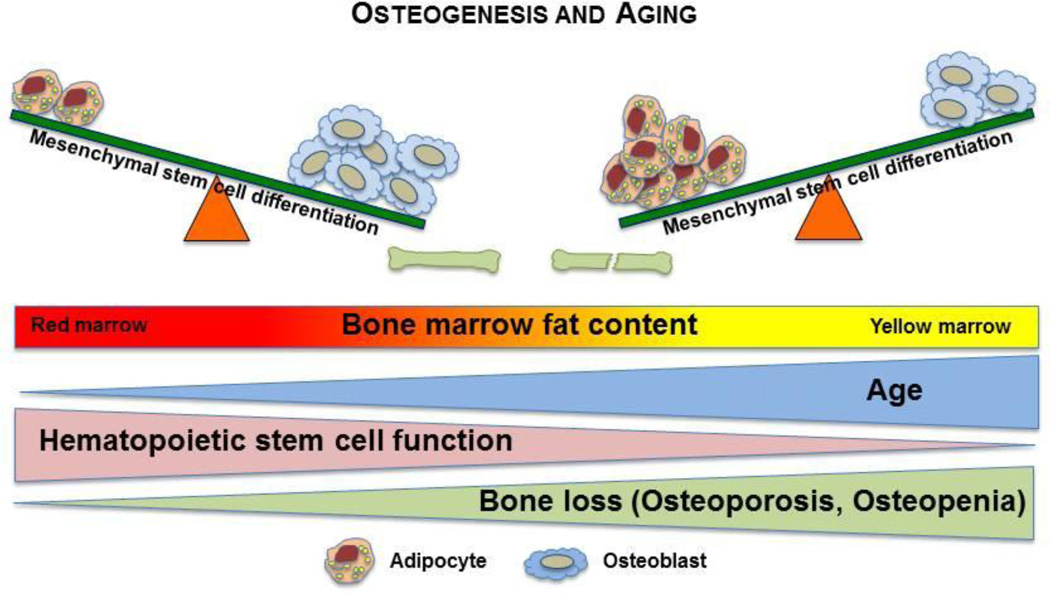
There has been a recent explosion of interest in the interactions between bone, fat, and blood. We now understand that there is a balance between osteogenesis and adipogenesis that changes as we grow older. As the overall population ages, obesity rates increase and the incidence of osteoporosis and diabetes rises. At the same time, hematopoiesis in the elderly is not as robust as it is in younger individuals suggesting that the hematopoietic microenvironment mediates a negative regulatory effect on hematopoiesis (Figure 1 is a cartoon model illustrating these changes with age). In this review, we examined many of the complex mechanisms governing the relationships between bone, fat, and blood formation and how the aging process changes these processes; however, there is still much that remains unknown. Understanding how these tissues develop and change with time and defining how OB and AP interact during aging to impact bone health and hematopoiesis will be critically important to developing new and better therapies for disorders of bone, metabolism and hematopoiesis in the elderly.
Figure 1.
Bone marrow microenvironment changes with aging. MSCs, which reside in the bone marrow, are common ancestors of both APs and OBs. In youth, MSC differentiation favors osteoblastogenesis; however; in aging, this balance progressively leans towards adipogenesis. This mirrors the gradual accumulation of APs within the bone marrow. Concurrently, HSC function, and subsequently hematopoiesis, declines with aging.
Footnotes
Disclosure
M Bethel: NIH training grant; BR Chitteti: none; EF Srour: none; and MA Kacena: institution NIH grant.
Contributor Information
Monique Bethel, Postdoctoral Fellow, Department of Orthopaedic Surgery, Indiana University School of Medicine, 1120 South Drive, FH 115, Indianapolis, IN 46202, 317-278-2804 (phone), 317-278-9568 (fax), mobethel@iupui.edu.
Brahmananda R. Chitteti, Postdoctoral Fellow, Department of Medicine. Indiana University School of Medicine, 980 W. Walnut Street, R3-C356, Indianapolis, IN 46202, 317-274-0352 (phone), 317-274-0396 (fax), bchittet@iupui.edu.
Edward F. Srour, Robert J. and Annie S. Rohn Professor of Leukemia Research, Departments of Medicine, Pediatrics, Microbiology and Immunology. Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, 980 W. Walnut Street, R3-C312, Indianapolis, IN 46202, 317-274-0343 (phone), 317-274-0396 (fax), esrour@iupui.edu.
Melissa A. Kacena, Assistant Professor, Department of Orthopaedic Surgery, Indiana University School of Medicine, 1120 South Drive, FH 115H, Indianapolis, IN 46202, 317-2783482 (phone), 317-278-9568 (Fax), mkacena@iupui.edu.
References
Papers of particular interest, published recently, have been highlighted as:
•Of importance
••Of major importance
- 1.Zafon C. Oscillations in total body fat content through life: an evolutionary perspective. Obes Rev. 2007;8:525–530. doi: 10.1111/j.1467-789X.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- 2.Abrams SA. Normal acquisition and loss of bone mass. Horm Res. 2003;60(Suppl 3):71–76. doi: 10.1159/000074505. [DOI] [PubMed] [Google Scholar]
- 3.Warren LA, Rossi DJ. Stem cells and aging in the hematopoietic system. Mech Ageing Dev. 2009;130:46–53. doi: 10.1016/j.mad.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Justesen J, Stenderup K, Ebbesen EN, et al. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 5.Giordano A, Cesari P, Capparuccia L, et al. Sema3A and neuropilin-1 expression and distribution in rat white adipose tissue. J Neurocytol. 2003;32:345–352. doi: 10.1023/B:NEUR.0000011328.61376.bb. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 7.Otto F, Thornell AP, Crompton T, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 8.Marie PJ. Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys. 2008;473:98–105. doi: 10.1016/j.abb.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 9.Fajas L, Fruchart JC, Auwerx J. Transcriptional control of adipogenesis. Curr Opin Cell Biol. 1998;10:165–173. doi: 10.1016/s0955-0674(98)80138-5. [DOI] [PubMed] [Google Scholar]
- 10.Lin GL, Hankenson KD. Integration of, BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem. 2011;112:3491–3501. doi: 10.1002/jcb.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowers RR, Lane MD. Wnt signaling and adipocyte lineage commitment. Cell Cycle. 2008;7:1191–1196. doi: 10.4161/cc.7.9.5815. [DOI] [PubMed] [Google Scholar]
- 12.Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Farmer SR. Regulating the balance between peroxisome proliferator-activated receptor gamma and beta-catenin signaling during adipogenesis. A glycogen synthase kinase 3beta phosphorylation-defective mutant of beta-catenin inhibits expression of a subset of adipogenic genes. J Biol Chem. 2004;279:45020–45027. doi: 10.1074/jbc.M407050200. [DOI] [PubMed] [Google Scholar]
- 14.Cawthon PM. Gender differences in osteoporosis and fractures. Clin Orthop Relat Res. 2011;469:1900–1905. doi: 10.1007/s11999-011-1780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seeman E. Invited Review: Pathogenesis of osteoporosis. J Appl Physiol. 2003;95:2142–2151. doi: 10.1152/japplphysiol.00564.2003. [DOI] [PubMed] [Google Scholar]
- 16.Modder UI, Roforth MM, Nicks KM, et al. Characterization of mesenchymal progenitor cells isolated from human bone marrow by negative selection. Bone. 2012;50:804–810. doi: 10.1016/j.bone.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Ippolito G, Schiller PC, Ricordi C, et al. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 18.Zhou S, Greenberger JS, Epperly MW, et al. Age-related intrinsic changes in human bone-marrow- derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishida S, Endo N, Yamagiwa H, et al. Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J Bone Miner Metab. 1999;17:171–177. doi: 10.1007/s007740050081. [DOI] [PubMed] [Google Scholar]
- 20.Kuznetsov SA, Mankani MH, Bianco P, et al. Enumeration of the colony-forming unitsfibroblast from mouse and human bone marrow in normal and pathological conditions. Stem Cell Res. 2009;2:83–94. doi: 10.1016/j.scr.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stenderup K, Justesen J, Clausen C, et al. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Rauner M, Sipos W, Pietschmann P. Age-dependent Wnt gene expression in bone and during the course of osteoblast differentiation. Age (Dordr) 2008;30:273–282. doi: 10.1007/s11357-008-9069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Essers MA, de Vries-Smits LM, Barker N, et al. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science. 2005;308:1181–1184. doi: 10.1126/science.1109083. [DOI] [PubMed] [Google Scholar]
- 24.Almeida M, Han L, Martin-Millan M, et al. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem. 2007;282:27298–27305. doi: 10.1074/jbc.M702811200. [DOI] [PubMed] [Google Scholar]
- 25.Chang S, Multani AS, Cabrera NG, et al. Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nat Genet. 2004;36:877–882. doi: 10.1038/ng1389. [DOI] [PubMed] [Google Scholar]
- 26.Du X, Shen J, Kugan N, et al. Telomere shortening exposes functions for the mouse Werner and Bloom syndrome genes. Mol Cell Biol. 2004;24:8437–8446. doi: 10.1128/MCB.24.19.8437-8446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pignolo RJ, Suda RK, McMillan EA, et al. Defects in telomere maintenance molecules impair osteoblast differentiation and promote osteoporosis. Aging Cell. 2008;7:23–31. doi: 10.1111/j.1474-9726.2007.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saeed H, Abdallah BM, Ditzel N, et al. Telomerase-deficient mice exhibit bone loss owing to defects in osteoblasts and increased osteoclastogenesis by inflammatory microenvironment. J Bone Miner Res. 2011;26:1494–1505. doi: 10.1002/jbmr.349. [DOI] [PubMed] [Google Scholar]
- 29.Simonsen JL, Rosada C, Serakinci N, et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20:592–596. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]
- 30. Wang H, Chen Q, Lee SH, et al. Impairment of osteoblast differentiation due to proliferation-independent telomere dysfunction in mouse models of accelerated aging. Aging Cell. 2012;11:704–713. doi: 10.1111/j.1474-9726.2012.00838.x. This study highlights how processes common in aging males and females can inhibit osteoblastogenesis and thus bone formation, helping shift the focus away from the estrogen deficiency model of osteoporosis.
- 31.Ding SL, Shen CY. Model of human aging: recent findings on Werner's and Hutchinson- Gilford progeria syndromes. Clin Interv Aging. 2008;3:431–444. doi: 10.2147/cia.s1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivas D, Li W, Akter R, et al. Accelerated features of age-related bone loss in zmpste24 metalloproteinase-deficient mice. J Gerontol A Biol Sci Med Sci. 2009;64:1015–1024. doi: 10.1093/gerona/glp089. [DOI] [PubMed] [Google Scholar]
- 33.Merideth MA, Gordon LB, Clauss S, et al. Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med. 2008;358:592–604. doi: 10.1056/NEJMoa0706898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akter R, Rivas D, Geneau G, et al. Effect of lamin A/C knockdown on osteoblast differentiation and function. J Bone Miner Res. 2009;24:283–293. doi: 10.1359/jbmr.081010. [DOI] [PubMed] [Google Scholar]
- 35.Liu LF, Shen WJ, Zhang ZH, et al. Adipocytes decrease Runx2 expression in osteoblastic cells: roles of PPARgamma and adiponectin. J Cell Physiol. 2010;225:837–845. doi: 10.1002/jcp.22291. [DOI] [PubMed] [Google Scholar]
- 36.Mackall JC, Student AK, Polakis SE, et al. Induction of lipogenesis during differentiation in a "preadipocyte" cell line. J Biol Chem. 1976;251:6462–6464. [PubMed] [Google Scholar]
- 37.Liu P, Oyajobi BO, Russell RG, et al. Regulation of osteogenic differentiation of human bone marrow stromal cells: interaction between transforming growth factor-beta and 1,25(OH)(2) vitamin D(3) In vitro. Calcif Tissue Int. 1999;65:173–180. doi: 10.1007/s002239900678. [DOI] [PubMed] [Google Scholar]
- 38.Zhou S, LeBoff MS, Glowacki J. Vitamin D metabolism and action in human bone marrow stromal cells. Endocrinology. 2010;151:14–22. doi: 10.1210/en.2009-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geng S, Zhou S, Glowacki J. Age-related decline in osteoblastogenesis and 1alpha-hydroxylase/ CYP27B1 in human mesenchymal stem cells: stimulation by parathyroid hormone. Aging Cell. 2011;10:962–971. doi: 10.1111/j.1474-9726.2011.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi K, Tsuboyama T, Matsushita M, et al. Modification of strain-specific femoral bone density by bone marrow-derived factors administered neonatally: a study on the spontaneously osteoporotic mouse, SAMP6. Bone Miner. 1994;24:245–255. doi: 10.1016/s0169-6009(08)80141-9. [DOI] [PubMed] [Google Scholar]
- 41.Kajkenova O, Lecka-Czernik B, Gubrij I, et al. Increased adipogenesis and myelopoiesis in the bone marrow of SAMP6, a murine model of defective osteoblastogenesis and low turnover osteopenia. J Bone Miner Res. 1997;12:1772–1779. doi: 10.1359/jbmr.1997.12.11.1772. [DOI] [PubMed] [Google Scholar]
- 42.Moerman EJ, Teng K, Lipschitz DA, et al. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sohal RS, Allen RG. Relationship between metabolic rate, free radicals, differentiation and aging: a unified theory. Basic Life Sci. 1985;35:75–104. doi: 10.1007/978-1-4899-2218-2_4. [DOI] [PubMed] [Google Scholar]
- 44. Almeida M, Ambrogini E, Han L, et al. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol Chem. 2009;284:27438–27448. doi: 10.1074/jbc.M109.023572. This study examines how oxidative stresses may be contributing to osteoporosis, which will help address the disease in men and continued bone loss in women long after menopause.
- 45.Morse D, Choi AM. Heme oxygenase-1: the "emerging molecule" has arrived. Am J Respir Cell Mol Biol. 2002;27:8–16. doi: 10.1165/ajrcmb.27.1.4862. [DOI] [PubMed] [Google Scholar]
- 46.Vanella L, Kim DH, Asprinio D, et al. HO-1 expression increases mesenchymal stem cell-derived osteoblasts but decreases adipocyte lineage. Bone. 2010;46:236–243. doi: 10.1016/j.bone.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keane EM, Healy M, O'Moore R, et al. Hypovitaminosis D in the healthy elderly. Br J Clin Pract. 1995;49:301–303. [PubMed] [Google Scholar]
- 48.Kelly KA, Gimble JM. 1,25-Dihydroxy vitamin D3 inhibits adipocyte differentiation and gene expression in murine bone marrow stromal cell clones and primary cultures. Endocrinology. 1998;139:2622–2628. doi: 10.1210/endo.139.5.5970. [DOI] [PubMed] [Google Scholar]
- 49.Duque G, Macoritto M, Kremer R. 1,25(OH)2D3 inhibits bone marrow adipogenesis in senescence accelerated mice (SAM-P/6) by decreasing the expression of peroxisome proliferator-activated receptor gamma 2 (PPARgamma2) Exp Gerontol. 2004;39:333–338. doi: 10.1016/j.exger.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Krum SA, Wend K. Estrogen Receptor Alpha Regulation of Bone Marrow Adipogenesis; Annual Meeting of the American Society of Bone and Mineral Research; 2012. [Google Scholar]
- 51.Saely CH, Geiger K, Drexel H. Brown versus white adipose tissue: a mini-review. Gerontology. 2012;58:15–23. doi: 10.1159/000321319. [DOI] [PubMed] [Google Scholar]
- 52. Krings A, Rahman S, Huang S, et al. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50:546–552. doi: 10.1016/j.bone.2011.06.016. Although this has not been confirmed in humans, this study demonstrates that bone marrow APs in mice are not homogenous and have characteristics of both white and brown adipose tissue, which may have implications in hematopoiesis.
- 53.Guralnik JM, Eisenstaedt RS, Ferrucci L, et al. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 54.Beghe C, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):3S–10S. doi: 10.1016/j.amjmed.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007;211:144–156. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens. 2007;70:179–189. doi: 10.1111/j.1399-0039.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 57.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 58.Henry CJ, Marusyk A, DeGregori J. Aging-associated changes in hematopoiesis and leukemogenesis: what's the connection? Aging (Albany NY) 2011;3:643–656. doi: 10.18632/aging.100351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Belaid-Choucair Z, Lepelletier Y, Poncin G, et al. Human bone marrow adipocytes block granulopoiesis through neuropilin-1-induced granulocyte colony-stimulating factor inhibition. Stem Cells. 2008;26:1556–1564. doi: 10.1634/stemcells.2008-0068. [DOI] [PubMed] [Google Scholar]
- 60.Belaid Z, Hubint F, Humblet C, et al. Differential expression of vascular endothelial growth factor and its receptors in hematopoietic and fatty bone marrow: evidence that neuropilin-1 is produced by fat cells. Haematologica. 2005;90:400–401. [PubMed] [Google Scholar]
- 61. Poncin G, Beaulieu A, Humblet C, et al. Characterization of spontaneous bone marrow recovery after sublethal total body irradiation: importance of the osteoblastic/adipocytic balance. PLoS One. 2012;7:e30818. doi: 10.1371/journal.pone.0030818. This study suggests that elevated Runx2 expression was important in initiating hematopoietic cell recovery following radiation, while PPARγ expression was suppressed as recovery progressed. Therefore, the lack of OB lineage cells (and surplus of APs) in aging may account, at least in part, for declining hematopoiesis in the elderly.
- 62. Naveiras O, Nardi V, Wenzel PL, et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. Here, the investigators found that APs can inhibit hematopoiesis at the level of the HSC, affecting all hematopoietic lineages.
- 63.Zhu RJ, Wu MQ, Li ZJ, et al. Hematopoietic recovery following chemotherapy is improved by BADGE-induced inhibition of adipogenesis. Int J Hematol. 2012 doi: 10.1007/s12185-012-1233-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 64.Spangrude GJ, Aihara Y, Weissman IL, et al. The stem cell antigens Sca-1 and Sca-2 subdivide thymic and peripheral T lymphocytes into unique subsets. J Immunol. 1988;141:3697–3707. [PubMed] [Google Scholar]
- 65.Chitteti BR, Cheng YH, Poteat B, et al. Impact of interactions of cellular components of the bone marrow microenvironment on hematopoietic stem and progenitor cell function. Blood. 2010;115:3239–3248. doi: 10.1182/blood-2009-09-246173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nishio M, Yoneshiro T, Nakahara M, et al. Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab. 2012;16:394–406. doi: 10.1016/j.cmet.2012.08.001. This study shows for the first time, a direct connection between human BAT and hematopoeisis. Murine bone marrow adipocytes have been shown to have characteristics of BAT which diminishes with aging. If the same is true in humans, this may also be an important factor in hematopoietic decline with aging.
- 67.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 68.Arai F, Hirao A, Suda T. Regulation of hematopoietic stem cells by the niche. Trends Cardiovasc Med. 2005;15:75–79. doi: 10.1016/j.tcm.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 69.Jung Y, Wang J, Song J, et al. Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood. 2007;110:82–90. doi: 10.1182/blood-2006-05-021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jung Y, Song J, Shiozawa Y, et al. Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche. Stem Cells. 2008;26:2042–2051. doi: 10.1634/stemcells.2008-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liao J, Hammerick KE, Challen GA, et al. Investigating the role of hematopoietic stem and progenitor cells in regulating the osteogenic differentiation of mesenchymal stem cells in vitro. J Orthop Res. 2011;29:1544–1553. doi: 10.1002/jor.21436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chitteti BR, Cheng YH, Streicher DA, et al. Osteoblast lineage cells expressing high levels of Runx2 enhance hematopoietic progenitor cell proliferation and function. J Cell Biochem. 2010;111:284–294. doi: 10.1002/jcb.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]