Table 2.
Calculated Activation Energies for ZnCl2-Catalyzed Cycloadditions of 3b with Disubstituted Furans.a
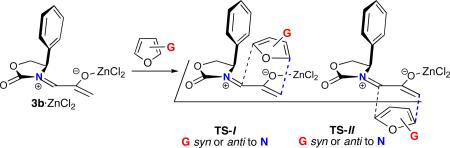
| ||||
|---|---|---|---|---|
| Reactants | ΔH‡ (ΔG‡) [ΔG‡soln] |
|||
| syn-I | anti-I | syn-II | anti-II | |
| 4 + 3b·ZnCl2 | –13.2 (5.2) [11.5] | –12.7 (4.9) [10.1] | –16.1 (2.2) [7.2] | –9.9 (6.8) [11.0] |
| 5 + 3b·ZnCl2 | –13.2 (4.3) [12.5] | –15.5 (3.6) [11.9] | –14.6 (2.6) [7.3] | –13.0 (5.6) [14.1] |
| 6 + 3b·ZnCl2 | –12.8 (3.9) [8.4] | –15.0 (2.3) [7.4] | –12.1 (3.6) [8.0] | –12.4 (3.9) [8.0] |
| 6 + 3c·ZnCl2 | –7.0 (9.7) [14.3] | –8.3 (9.0) [14.4] | –6.1 (9.6) [14.2] | –6.0 (10.4) [14.9] |
| 7 + 3b·ZnCl2 | –9.6 (8.4) [16.1] | –11.5 (4.8) [9.9] | –14.4 (3.8) [11.0] | –8.3 (7.0) [11.4] |
M06-2X/6-311+G(d,p)//B3LYP/6-31G(d)–LANL2DZ. Solution-phase data incorporate CPCM solvation energies in CH2Cl2, computed at the M06-2X/6-31G(d)–LANL2DZ level. ΔH† and ΔG‡ in kcal/mol at 298.15 K.
