Abstract
In the seminiferous epithelium of the mammalian testis, the most distinctive ultrastructure is the extensive bundles of actin filaments that lie near the Sertoli-spermatid interface and the Sertoli-Sertoli cell interface known as the apical ectoplasmic specialization (apical ES) and the basal ES, respectively. These actin filament bundles not only confer strong adhesion at these sites, they are uniquely found in the testis. Recent studies have shown that ES also confers spermatid and Sertoli cell polarity in the seminiferous epithelium during the epithelial cycle. While these junctions were first described in the 1970s, there are few functional studies in the literature to examine the regulation of these actin filament bundles. It is conceivable that these actin filament bundles at the ES undergo extensive re-organization to accommodate changes in location of developing spermatids during spermiogenesis as spermatids are transported across the seminiferous epithelium. Additionally, these actin filaments are rapidly reorganized during BTB restructuring to accommodate the transit of preleptotene spermatocytes across the barrier at stage VIII of the epithelial cycle. Thus, actin binding and regulatory proteins are likely involved in these events to confer changes in F-actin organization at these sites. Interestingly, there are no reports in the field to study these regulatory proteins until recently. Herein, we summarize some of the latest findings in the field regarding a novel actin cross-linker and actin-bundling protein called palladin. We also discuss in this opinion article the likely role of palladin in regulating actin filament bundles at the ES during spermatogenesis, highlighting the significant of palladin and how this protein is plausibly working in concert with other actin-binding/regulatory proteins and components of polarity proteins to regulate the cyclic events of actin organization and re-organization during the epithelial cycle of spermatogenesis. We also propose a hypothetic model by which palladin regulates ES restructuring during the epithelial cycle of spermatogenesis.
Keywords: testis, spermatogenesis, seminiferous epithelial cycle, F-actin, ectoplasmic specialization, tight junction, blood-testis barrier, Sertoli cells
Introduction
Palladin (Fig. 1), myotilin and myopalladin form a unique family of actin-binding proteins that were known to have a scaffolding function.1,2 Palladin was initially found to co-localize with α-actinin in stress fibers in cells, focal adhesions (also known as focal contacts) and cell-cell junctions,3 and also associated with α-actinin to organize actin-containing microfilaments in smooth muscle and nonmuscle cells,4 serving a scaffolding role in cytoskeleton. Unlike palladin, which is found in virtually all mammalian tissues and cells and localized to actin-based structures (e.g., stress fibers, focal adhesions),1,2,5,6 myopalladin is restricted to the heart and skeletal muscle,7 whereas myotilin is mostly expressed in skeletal muscle.8 Subsequent studies have shown that palladin, besides serving as a cytoskeletal scaffold, also binds tightly to Eps8 (epidermal growth factor receptor pathway substrate 8, an actin-barbed end capping and bundling protein), and it is also an actin cross-linker that binds to F-actin using its Ig-like (immunoglobulin-like) domains.9 Due to this unusual actin cross-linking activity and the fact that it is tightly associated with Eps8, it is becoming increasingly clear that palladin is a crucial molecule that confers and regulates actin filament bundles in mammalian cells, such as those found in the ectoplasmic specialization (ES) in the seminiferous epithelium of the mammalian testis.

Figure 1. A schematic drawing illustrating the functional domains of a 90 kDa isoform of palladin polypeptide. In the testis, the predominant form of palladin detected by immunoblotting is the 90 kDa isoform, which is composed of a protein-rich region (PR region) for protein-protein interactions near its N terminus, and three IgC2 domains close to its C terminus for binding to F-actin to confer its F-actin cross-linking and F-actin bundling activity.
Three isoforms of palladin are found in mammalian cells to date; however, the 90 kDa isoform is the predominantly palladin in the rat testis (Fig. 1).10 The 90 kDa palladin polypeptide possesses three distinctive Ig-like domains near its C terminus, which confers actin-binding activity,5 with a proline-rich (PR) region near its N terminus (Fig. 1).5 The PR motif in palladin is similar to the motif found in zyxin and vinculin, which are adaptor proteins found in the rat testis,11,12 and the PR motif is also the binding site for VASP (vasodilator-stimulated phosphoprotein).13 Other studies have shown that the PR motif is crucial to induce interactions with SH3 domains, PDZ (postsynaptic density disc-large/ZO-1) domains, WW (tryptophan-tryptophan) domains and EH (Eps15 homology) domains of other proteins,14 thereby, palladin possesses the ability to recruit other proteins to the actin filament bundles in mammalian cells to affect a number of cell functions. Indeed, besides interacting with α-actinin,15 palladin has been shown to interact with the SH3 domain of c-Src.16 Recent studies have shown that c-Yes, a member of the Src kinase family (SKF), is also an important regulator of F-actin in the testis,17,18 illustrating palladin can be activated by members of the SKF to modulate its actin bundling activity. Furthermore, palladin also interacts specifically with Akt1 but not Akt219,20 (note: Akt, also known as protein kinase B, PKB, which is also a component of the F-actin-rich ultrastructures in the testis21). The physiological significance of palladin was shown in studies using the genetic model of palladin−/−-knockout (KO) mice since these mice displayed embryonic lethality, died at around embryonic age E15.5 d, then developed cranial neural tube closure defects, due to the inability of forming stress fibers and cell adhesion in the neural tissue to elicit cell migration necessary to develop the cranial neural tube.22 Palladin−/− mouse embryos also displayed defects in erythropoiesis in fetal liver.23
Recent studies have also shown that palladin is involved in carcinogenesis. Since extensive cell migration takes place in tumorigenesis, such as cancer invasion and metastasis,24,25 and overexpression of palladin indeed was shown to activate tumor invasion.2,26,27 Also, activation of palladin was recently shown to be mediated by ERK (extracellular signal-regulated kinase) by phosphorylating palladin at Ser77 and Ser197, which induced anti-migratory activities in cancer cells.28
Actin filaments in the seminiferous epithelium of adult rat testis during the epithelial cycle—the likely role of palladin in actin re-organization
In the mammalian testis, such as rodents and humans, one of the most noticeable features in the seminiferous epithelium is the presence of extensive filament bundles that lie perpendicular to the opposing plasma membranes of: (1) Sertoli cells at the blood-testis barrier (BTB) or (2) Sertoli and elongating/elongated spermatids.29-32 These actin filament bundles are sandwiched in-between the opposing plasma membrane in (1 and 2) and the cisternae of endoplasmic reticulum in the Sertoli cell and are known as the basal and the apical ectoplasmic specialization (ES), respectively.29-32 In short, the ES is a testis-specific atypical adherens junction (AJ),33 since the ES is only found in the seminiferous epithelium of mammalian testes. Also, the integral membrane proteins at the ES that are usually restricted either to tight junctions [TJs; e.g., coxsackievirus and adenovirus receptor (CAR), junctional adhesion molecule-A (JAM-A), JAM-B, JAM-C, claudin-5], AJs (e.g., N-cadherin, nectin-2, nectin-3), gap junctions (e.g., connexin-33, connexin-43), focal adhesion complexes (e.g., integrins, laminins) and desmosomes (e.g., desmoglein-2) are all found in the ES: either at the apical or basal ES; and, as such, making the ES a hybrid cell-adhesion junction.31,33-35 While other studies have demonstrated that actin filament bundles are crucial to confer the unusual adhesive strength to the apical ES as well as the basal ES at the BTB,36,37 and their involvement in spermatid transport during spermiogenesis,38,39 virtually no functional studies were found in the literature to study actin-based cytoskeleton until recently.30,40-42 It is increasingly clear that the highly restrictive and stage-specific spatiotemporal expression of Eps8 (an actin-bundling and barbed end capping protein that confers actin filament bundles at the ES),43 Arp3 (actin-related protein 3, which together with Arp2 forms the Arp2/3 complex that mediates branched actin polymerization, thereby converting the actin filaments from a “bundled” to a “de-bundled” and “branched” actin network state, destabilizing the ES, necessary to allow spermatid transport across the epithelium during spermiogenesis and the transit of preleptotene spermatocytes at the BTB)44 and drebrin E (an actin-binding protein that recruits Arp3 to the ES to induce branched actin network),45 which together with polarity proteins (e.g., Par3, Par6, 14–3-3, Cdc43, Scribble, Lgl, Dlg)46-49 and non-receptor protein tyrosine kinases (e.g., c-Yes, c-Src),17,18 are involved in regulating the transformation of actin between its “bundled” and “de-bundled” state as illustrated in an in vivo model using adjudin, mimicking junction restructuring at spermiation.42
In order to provide a thorough understanding on the regulation of actin dynamics in the testis, we thought it necessary to identify other potential players in this event. In our quest of searching for actin-bundling proteins besides Eps8, filamin A (an actin cross-linking protein) was found in the testis; however, its expression and function were shown to be limited to the assembly of the BTB during testicular maturation following puberty.50,51 It is likely that filamin A may also regulate junction restructuring events during spermatogenesis in adulthood; however, its steady-state level, both its protein and mRNA in the adult testis is only ~5–10% of that of immature rat testes and it is critically involved in the assembly of the BTB at puberty,50 thus, it is unlikely that this actin cross-linker plays a critical role in regulating BTB dynamics during spermatogenesis in adult rat testes.
The 90 kDa isoform of palladin, an actin cross-linker and an actin-bundling protein, was detected both at the apical ES and at the basal ES at the BTB in adult rat testes, highly expressed by Sertoli and germ cells.10 Furthermore, palladin was almost exclusively localized to the apical ES and the basal ES at the BTB,10 the F-actin-rich ultrastructures in the seminiferous epithelium that require changes in their organization from their “bundled” to “de-bundled” configuration and vice versa during the epithelial cycle. Also, palladin was also detected at the tunica propria, apparently associated with peritubular myoid cells.10 Most notably, the localization of palladin at the apical ES surrounding the heads of spermatids changes considerably during spermiogenesis, such as from stages I‒II, IV‒V, VI‒VII, VIII and XII‒XIII when step 15–16, 17, 18–19, 19 and 12–13, respectively, are found in the adluminal compartment (Fig. 2), reflecting the need of the apical ES to undergo extensive re-organization of the actin filament bundles at the site to accommodate spermatid transport across the epithelium, which also coupled with changes in the morphology of spermatid head (e.g., packaging of the genetic materials in the spermatid nucleus in the head region, formation of acrosome) and spermatid polarity. Thus, palladin is working in conjunction with other actin-binding and regulatory proteins, such as Eps 8, Arp2/3 complex, drebrin E, filamin A and perhaps other yet-to-be identified actin regulatory proteins, to confer changes of the actin filament bundles by altering between the “bundled” and “de-bundled” configuration. For instance, spermatids have to be transported from near the basement membrane toward the adluminal edge of the tubule at stages I‒IV, but also be transported back to the basal compartment by almost touching the Sertoli cell nucleus located in the basal compartment at stage V, and then transported back up to the adluminal compartment at stages VI‒VII until they line up at the luminal edge near the tubule lumen at early stage VIII with their heads pointing toward the basement membrane and their tails to the tubule lumen in highly polarized fashion to prepare for spermiation.52-54
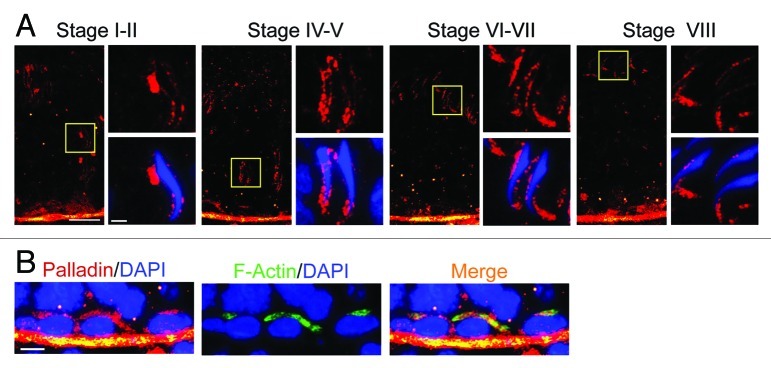
Figure 2. Stage-specific localization and expression of palladin in the seminiferous epithelium of adult rat testes. Palladin (red fluorescence) was localized to the tunica propria, associated with peritubular myoid cells and also near the basement membrane, consistent with its localization at the BTB. (A) Most notably, palladin was localized at the apical ES, surrounding the head of elongating spermatids, in step 15–19 spermatids at stage I‒VIII of the epithelial cycle. More importantly, its localization at the apical ES altered during the progression of spermiogenesis. For instance, at stage I‒V, palladin was found to surround the entire spermatid head, but it shifted mostly to the tip of the spermatid head beginning at stage VII, and most obvious at stage VIII, illustrating that palladin is no longer needed to confer the actin filament bundles at stage VIII in preparation for the release of sperm at spermiation. Immunofluorescence microscopy was performed as described in reference 10 using a rabbit anti-palladin antibody (Protein Tech, Cat. # 10853–1-AP, working dilution, 1:100), spermatid nuclei were visualized by DAPI (4’,6-diamidino-2-phenylindole) staining. For each staged tubule, the “yellow” boxed area on the left was magnified and shown on the right, scale bar = 50 μm in the micrograph on the left, and scale bar = 10 μm in the micrograph enlarged on the right, which apply to corresponding micrographs in all other stages. These observations support the notion that palladin is involved in conferring changes in the organization of F-actin filaments from their “bundles” and “de-bundled” configuration to facilitate spermatid transport across the seminiferous epithelium as well as changes in the shape/morphology of the spermatid head, as well as spermatid polarity during spermiogenesis. (B) Palladin (red fluorescence) was also found to co-localize with F-actin at the BTB besides the tunica propriate (F-actin was detected by using phalloidin-FITC) (Sigma-Aldrich, Cat. # P5282; working dilution, 1:70). Bar = 10 μm in the micrograph on the left, which apply to corresponding micrographs in this panel.
It was also shown that a knockdown of palladin by RNAi using specific siRNA duplexes in Sertoli cells cultured in vitro with an established functional TJ-permeabiltiy barrier that mimicked the BTB in vivo, the silencing of palladin by ~60% was found to impede the actin filaments in Sertoli cells in which the typical actin filaments were no longer seen in these cells, they were either mis-aligned or truncated, failing to form actin filament bundles, thereby perturbing the Sertoli cell TJ-permeability barrier function.10 This loss of actin filament network in the Sertoli cell cytosol also impeded the proper distribution of ZO-1 and α-catenin at the cell-cell interface. Furthermore, a knockdown of palladin in the testis in vivo by RNAi was found to impede spermatid adhesion in which elongated spermatids failed to undergo spermiation so that defects of were defected in which spermatids were entrapped in the seminiferous epithelium in stage IX‒X tubules.
It is likely that palladin is working with Eps8 to confirm integrity of the actin filament bundles at the apical ES, and the stage-specific changes on the expression of palladin around the apical ES during the epithelial cycle as illustrated in Figure 2 may be crucial and necessary to alter the configuration of actin filament bundles surrounding the spermatid head to allow the transport of spermatid across the seminiferous epithelium during spermiogenesis. It is also plausible that other proteins, such as Par6,46 Scribble49 and c-Yes,17,55 which also display stage-specific and spatiotemporal expression at the ES, may also be involved in the apical ES restructuring. Furthermore, a recent report has demonstrated that the restrictive expression of two isoforms of FAK, namely p-FAK-Tyr407 and p-FAK-Tyr397, are having antagonistic effects on the Sertoli cell TJ barrier function via their effects on the actin filament dynamics at the basal ES.56 It is possible that the restrictive expression of these two isoforms of FAK at the ES affects the intriguing spatiotemporal expression of the actin regulatory proteins that confer “bundling” (e.g., palladin, Eps8) and “de-bundling” (e.g., the Arp2/3 complex) of the F-actin at the ES, so that actin filament bundles can be rapidly modified surrounding the spermatid head to accommodate their transport along the seminiferous epithelium during spermiogenesis.
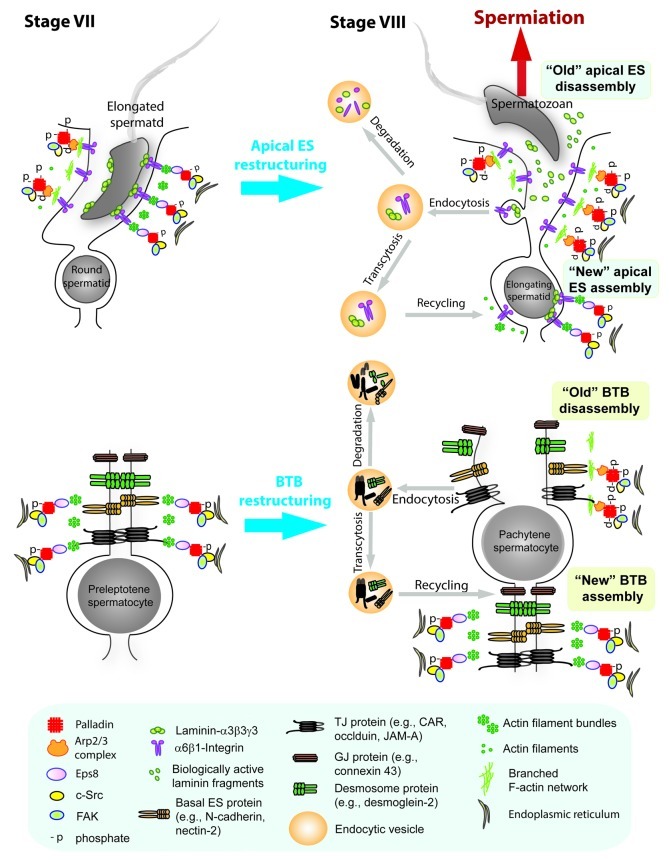
Based on recent findings that both Eps8, Arp3 and c-Src are binding partners of palladin, which coupled with the observations regarding the stage-specific and spatiotemporal expression of palladin,10 Eps8,43 Arp344 and c-Src,18 we provide a hypothetical model by which palladin regulates ES restructuring during the epithelial cycle of spermatogenesis (Fig. 3). In short, at stage VII of the epithelial cycle when the apical and the basal ES are intact, c-Src (and also FAK since it is an integrated component of both the apical and the basal ES56-59) provides proper phosphorylation to activate palladin to exert its actin cross-linking and bundling activity; also, palladin recruits Eps8 to the site to maintain the actin filaments at the ES in their “bundled” configuration (see left panel of Fig. 3). At stage VIII of the epithelial cycle, cSrc/FAK inactivates palladin via phosphorylation to “disable” its actin cross-linking and bundling activity, also, instead of recruiting Eps8, Arp2/3 complex is predominantly recruited to the ES, this, in turn, induces branched F-actin, converting the “bundled” actin filaments to a “un-bundled” configuration (see right panel of Fig. 3). These changes in the underlying actin cytoskeleton also contribute to an increase in endocytic vesicle-mediated protein endocytosis, causing protein transcytosis and recycling to assemble “new” apical ES and also “new” BTB.
Figure 3. A schematic drawing illustrating the mechanism by which palladin regulates ES restructuring during the epithelial cycle of spermatogenesis. Left panel shows the apical ES (upper) and the basal ES at the BTB (lower) at stage VII of the epithelial cycle when the ES is intact, maintained by adhesion proteins at the apical ES and basal ES (and also proteins of the TJ, GJ and desmosome at the BTB) to confer spermatid adhesion and the integrity of the BTB, respectively, since these proteins are anchored to the underlying actin filament bundles at both sites. The ES integrity is made possible in which c-Src and FAK maintains the proper phosphorylation status on palladin, which exerts its actin cross-linking and actin-bundling activity; also, palladin recruits Eps8 to the apical ES and the basal ES to maintain and strengthen the integrity of the actin filament bundles. On the right panel is the seminiferous epithelium at stage VIII of the epithelial cycle in which both the apical ES (upper) and the basal ES/BTB (lower) undergo restructuring to facilitate the release of spermatzoa at spermiation and the transit of preleptotene spermatocytes across the BTB. This is made possible in which c-Src and FAK phosphorylate palladin to inactivate its actin cross-linking and bundling intrinsic activity, such changes in phosphorylation status also reduce the binding affinity of palladin to Eps8, instead, more Arp3 is recruited to the apical and the basal ES, which induce branched actin polymerization, destabilizing the ES at both sites to facilitate spermiation and the transit of preleptotene spermatocytes at the BTB. The segregation of laminin chains from the integrin receptors at the apical ES (note: laminin- α3β3γ3-α6β1-integrin is an adhesion protein complex at the apical ES) also induces the generation of biologically active laminin fragments mediated via the action of MMP-2, which can further potentiate BTB restructuring as reported earlier.60,61 Changes in actin filament organization at the ES at both sites from the “bundled” to the “de-bundled” configuration also favor endocytic vesicle-medicated protein endocytosis, transcytosis and recycling to assemble “new” apical ES and “new” basal ES/BTB during disassembly of the “old” apical ES and the “old” basal ES/BTB. However, “aged” proteins undergo endosome-/ubiquitin-mediated intracellular degradation instead of being recycled. In short, this model illustrates the physiological significance of palladin in the restructuring of the apical and the basal ES during the epithelial cycle of spermatogenesis via its interaction with Eps8, Arp3 and c-Src.
Concluding Remarks and Future Perspectives
Collectively, the recent findings on palladin in the adult rat testis during the epithelial cycle have illustrated that palladin is an important regulator of the actin filament bundles at the ES, both at the Sertoli-spermatid interface at the apical ES and also the Sertoli-Sertoli cell interface at the basal ES in the BTB. Analogous to Eps 8,43 Arp3,44 drebrin E45 and filamin A,50 palladin also displays stage-specific and spatiotemporal expression in the epithelium in particular at the apical ES during the epithelial cycle in the rat testis. Furthermore, the unique localization pattern of palladin at the apical ES during the epithelial cycle also reflects its likely involvement in the cyclic events of apical ES restructuring in which actin filament bundles undergo complex re-organization to facilitate the timely transport of spermatid across the epithelium. These recent findings on palladin, in conjunction with other functional studies conducted on palladin in other epithelia as briefly described herein, thus help us to provide a hypothetical model depicted in Figure 3 by which palladin regulates ES restructuring during the epithelial cycle of spermatogenesis. This model also provides the basis of performing additional functional experiments in future studies. It is likely that these actin regulatory proteins are working in concert with different polarity proteins46-49 in the testis to confer actin re-organization during spermatogenesis.
Acknowledgments
Studies from the authors’ laboratory were supported by grants from the National Institutes of Health (NICHD, R01 HD056034 to C.Y.C.; U54 HD029990 Project 5 to C.Y.C.).
Footnotes
Previously published online: www.landesbioscience.com/journals/spermatogenesis/article/23473
References
- 1.Otey CA, Rachlin A, Moza M, Arneman D, Carpen O. The palladin/myotilin/myopalladin family of actin-associated scaffolds. Int Rev Cytol. 2005;246:31–58. doi: 10.1016/S0074-7696(05)46002-7. [DOI] [PubMed] [Google Scholar]
- 2.Jin L. The actin associated protein palladin in smooth muscle and in the development of diseases of the cardiovasculature and in cancer. J Muscle Res Cell Motil. 2011;32:7–17. doi: 10.1007/s10974-011-9246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parast MM, Otey CA. Characterization of palladin, a novel protein localized to stress fibers and cell adhesions. J Cell Biol. 2000;150:643–56. doi: 10.1083/jcb.150.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mykkänen OM, Grönholm M, Rönty M, Lalowski M, Salmikangas P, Suila H, et al. Characterization of human palladin, a microfilament-associated protein. Mol Biol Cell. 2001;12:3060–73. doi: 10.1091/mbc.12.10.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goicoechea SM, Arneman D, Otey CA. The role of palladin in actin organization and cell motility. Eur J Cell Biol. 2008;87:517–25. doi: 10.1016/j.ejcb.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rachlin AS, Otey CA. Identification of palladin isoforms and characterization of an isoform-specific interaction between Lasp-1 and palladin. J Cell Sci. 2006;119:995–1004. doi: 10.1242/jcs.02825. [DOI] [PubMed] [Google Scholar]
- 7.Bang ML, Mudry RE, McElhinny AS, Trombitás K, Geach AJ, Yamasaki R, et al. Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J Cell Biol. 2001;153:413–27. doi: 10.1083/jcb.153.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salmikangas P, Mykkänen OM, Grönholm M, Heiska L, Kere J, Carpén O. Myotilin, a novel sarcomeric protein with two Ig-like domains, is encoded by a candidate gene for limb-girdle muscular dystrophy. Hum Mol Genet. 1999;8:1329–36. doi: 10.1093/hmg/8.7.1329. [DOI] [PubMed] [Google Scholar]
- 9.Dixon RD, Arneman DK, Rachlin AS, Sundaresan NR, Costello MJ, Campbell SL, et al. Palladin is an actin cross-linking protein that uses immunoglobulin-like domains to bind filamentous actin. J Biol Chem. 2008;283:6222–31. doi: 10.1074/jbc.M707694200. [DOI] [PubMed] [Google Scholar]
- 10.Qian X, Mruk DD, Wong EWP, Lie PPY, Cheng CY. Palladin is a regulator of actin filament bundles at the ectoplasmic specialization in the rat testis. 2013 doi: 10.1210/en.2012-2269. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee NPY, Mruk DD, Conway AM, Cheng CY. Zyxin, axin, and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl. 2004;25:200–15. doi: 10.1002/j.1939-4640.2004.tb02780.x. [DOI] [PubMed] [Google Scholar]
- 12.Young JS, Vogl AW. Focal adhesion proteins Zyxin and Vinculin are co-distributed at tubulobulbar complexes. Spermatogenesis. 2012;2:63–8. doi: 10.4161/spmg.19391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boukhelifa M, Parast MM, Bear JE, Gertler FB, Otey CA. Palladin is a novel binding partner for Ena/VASP family members. Cell Motil Cytoskeleton. 2004;58:17–29. doi: 10.1002/cm.10173. [DOI] [PubMed] [Google Scholar]
- 14.Kay BK, Williamson MP, Sudol M. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000;14:231–41. [PubMed] [Google Scholar]
- 15.Rönty M, Taivainen A, Moza M, Otey CA, Carpén O. Molecular analysis of the interaction between palladin and alpha-actinin. FEBS Lett. 2004;566:30–4. doi: 10.1016/j.febslet.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Rönty M, Taivainen A, Heiska L, Otey C, Ehler E, Song WK, et al. Palladin interacts with SH3 domains of SPIN90 and Src and is required for Src-induced cytoskeletal remodeling. Exp Cell Res. 2007;313:2575–85. doi: 10.1016/j.yexcr.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao X, Mruk DD, Lee WM, Cheng CY. c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes. Int J Biochem Cell Biol. 2011;43:651–65. doi: 10.1016/j.biocel.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao X, Mruk DD, Cheng FL, Cheng CY. c-Src and c-Yes are two unlikely partners of spermatogenesis and their roles in blood-testis barrier dynamics. Adv Exp Med Biol. 2012;763:295–317. doi: 10.1007/978-1-4614-4711-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chin YR, Toker A. The actin-bundling protein palladin is an Akt1-specific substrate that regulates breast cancer cell migration. Mol Cell. 2010;38:333–44. doi: 10.1016/j.molcel.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chin YR, Toker A. Akt isoform-specific signaling in breast cancer: uncovering an anti-migratory role for palladin. Cell Adh Migr. 2011;5:211–4. doi: 10.4161/cam.5.3.15790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siu MKY, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–47. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- 22.Luo H, Liu X, Wang F, Huang Q, Shen S, Wang L, et al. Disruption of palladin results in neural tube closure defects in mice. Mol Cell Neurosci. 2005;29:507–15. doi: 10.1016/j.mcn.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Liu XS, Li XH, Wang Y, Shu RZ, Wang L, Lu SY, et al. Disruption of palladin leads to defects in definitive erythropoiesis by interfering with erythroblastic island formation in mouse fetal liver. Blood. 2007;110:870–6. doi: 10.1182/blood-2007-01-068528. [DOI] [PubMed] [Google Scholar]
- 24.Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol. 2011;27:185–211. doi: 10.1146/annurev-cellbio-092910-154216. [DOI] [PubMed] [Google Scholar]
- 25.Bravo-Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Curr Opin Cell Biol. 2012;24:277–83. doi: 10.1016/j.ceb.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brentnall TA, Lai LA, Coleman J, Bronner MP, Pan S, Chen R. Arousal of cancer-associated stroma: overexpression of palladin activates fibroblasts to promote tumor invasion. PLoS One. 2012;7:e30219. doi: 10.1371/journal.pone.0030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brentnall TA. Arousal of cacner-associated stromal fibroblasts: Palladin-activated fibroblasts promote tumor invasion. Cell Adhes Migr. 2012 doi: 10.4161/cam.21453. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asano E, Maeda M, Hasegawa H, Ito S, Hyodo T, Yuan H, et al. Role of palladin phosphorylation by extracellular signal-regulated kinase in cell migration. PLoS One. 2011;6:e29338. doi: 10.1371/journal.pone.0029338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- 30.Lie PPY, Mruk DD, Lee WM, Cheng CY. Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1581–92. doi: 10.1098/rstb.2009.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol. 2010;6:380–95. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng CY, Mruk DD. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit Rev Biochem Mol Biol. 2009;44:245–63. doi: 10.1080/10409230903061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim Biophys Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan HHN, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: a friend or a foe of spermatogenesis? Bioessays. 2007;29:36–48. doi: 10.1002/bies.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siu MKY, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. Bioessays. 2004;26:978–92. doi: 10.1002/bies.20099. [DOI] [PubMed] [Google Scholar]
- 36.Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. Strength measurement of the Sertoli-spermatid junctional complex. J Androl. 2005;26:354–9. doi: 10.2164/jandrol.04142. [DOI] [PubMed] [Google Scholar]
- 37.Russell LD, Goh JC, Rashed RMA, Vogl AW. The consequences of actin disruption at Sertoli ectoplasmic specialization sites facing spermatids after in vivo exposure of rat testis to cytochalasin D. Biol Reprod. 1988;39:105–18. doi: 10.1095/biolreprod39.1.105. [DOI] [PubMed] [Google Scholar]
- 38.Russell LD, Saxena NK, Turner TT. Cytoskeletal involvement in spermiation and sperm transport. Tissue Cell. 1989;21:361–79. doi: 10.1016/0040-8166(89)90051-7. [DOI] [PubMed] [Google Scholar]
- 39.Lee NPY, Cheng CY. Ectoplasmic specialization, a testis-specific cell-cell actin-based adherens junction type: is this a potential target for male contraceptive development? Hum Reprod Update. 2004;10:349–69. doi: 10.1093/humupd/dmh026. [DOI] [PubMed] [Google Scholar]
- 40.Cheng CY, Wong EW, Lie PP, Mruk DD, Xiao X, Li MW, et al. Polarity proteins and actin regulatory proteins are unlikely partners that regulate cell adhesion in the seminiferous epithelium during spermatogenesis. Histol Histopathol. 2011;26:1465–74. doi: 10.14670/hh-26.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng CY, Mruk DD. Actin binding proteins and spermiogenesis: Some unexpected findings. Spermatogenesis. 2011;1:99–104. doi: 10.4161/spmg.1.2.16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng CY, Lie PPY, Wong EWP, Mruk DD, Silvestrini B. Adjudin disrupts spermatogenesis via the action of some unlikely partners: Eps8, Arp2/3 complex, drebrin E, PAR6 and 14-3-3. Spermatogenesis. 2011;1:291–7. doi: 10.4161/spmg.1.4.18393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–67. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010;107:11411–6. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li MWM, Xiao X, Mruk DD, Lam YL, Lee WM, Lui WY, et al. Actin-binding protein drebrin E is involved in junction dynamics during spermatogenesis. Spermatogenesis. 2011;1:123–36. doi: 10.4161/spmg.1.2.16393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:9657–62. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong EWP, Sun S, Li MWM, Lee WM, Cheng CY. 14-3-3 Protein regulates cell adhesion in the seminiferous epithelium of rat testes. Endocrinology. 2009;150:4713–23. doi: 10.1210/en.2009-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong EWP, Mruk DD, Lee WM, Cheng CY. Regulation of blood-testis barrier dynamics by TGF-β3 is a Cdc42-dependent protein trafficking event. Proc Natl Acad Sci USA. 2010;107:11399–404. doi: 10.1073/pnas.1001077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su WH, Wong EWP, Mruk DD, Cheng CY. The Scribble/Lgl/Dlg polarity protein complex is a regulator of blood-testis barrier dynamics and spermatid polarity during spermatogenesis. Endocrinology. 2012;153:6041–53. doi: 10.1210/en.2012-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Su WH, Mruk DD, Lie PPY, Lui WY, Cheng CY. Filamin A is a regulator of blood-testis barrier assembly during postnatal development in the rat testis. Endocrinology. 2012;153:5023–35. doi: 10.1210/en.2012-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su WH, Mruk DD, Cheng CY. Filamin A: A regulator of blood-testis barrier assembly during post-natal development. Spermatogenesis. 2012;2:73–8. doi: 10.4161/spmg.20223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Donnell L, Nicholls PK, O’Bryan MK, McLachlan RI, Stanton PG. Spermiation: The process of sperm release. Spermatogenesis. 2011;1:14–35. doi: 10.4161/spmg.1.1.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hess RA, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- 54.Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev. 2008;60:146–80. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao X, Mruk DD, Cheng CY. c-Yes regulates cell adhesion at the apical ectoplasmic specialization-blood-testis barrier axis via its effects on protein recruitment and distribution. Am J Physiol Endocrinol Metab. 2012 doi: 10.1152/ajpendo.00422.2012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lie PPY, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci USA. 2012;109:12562–7. doi: 10.1073/pnas.1202316109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng CY, Mruk DD. Regulation of blood-testis barrier dynamics by focal adhesion kinase (FAK): an unexpected turn of events. Cell Cycle. 2009;8:3493–9. doi: 10.4161/cc.8.21.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siu MKY, Mruk DD, Lee WM, Cheng CY. Adhering junction dynamics in the testis are regulated by an interplay of β 1-integrin and focal adhesion complex-associated proteins. Endocrinology. 2003;144:2141–63. doi: 10.1210/en.2002-221035. [DOI] [PubMed] [Google Scholar]
- 59.Beardsley A, Robertson DM, O’Donnell L. A complex containing α6β1-integrin and phosphorylated focal adhesion kinase between Sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J Endocrinol. 2006;190:759–70. doi: 10.1677/joe.1.06867. [DOI] [PubMed] [Google Scholar]
- 60.Yan HHN, Mruk DD, Wong EWP, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:8950–5. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Su L., Mruk D.D., Lie P.P.Y., Silvestrini B., Cheng C.Y. A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats. Nat Communs. 2012;3:1185. doi: 10.1038/ncomms2171. [DOI] [PMC free article] [PubMed] [Google Scholar]