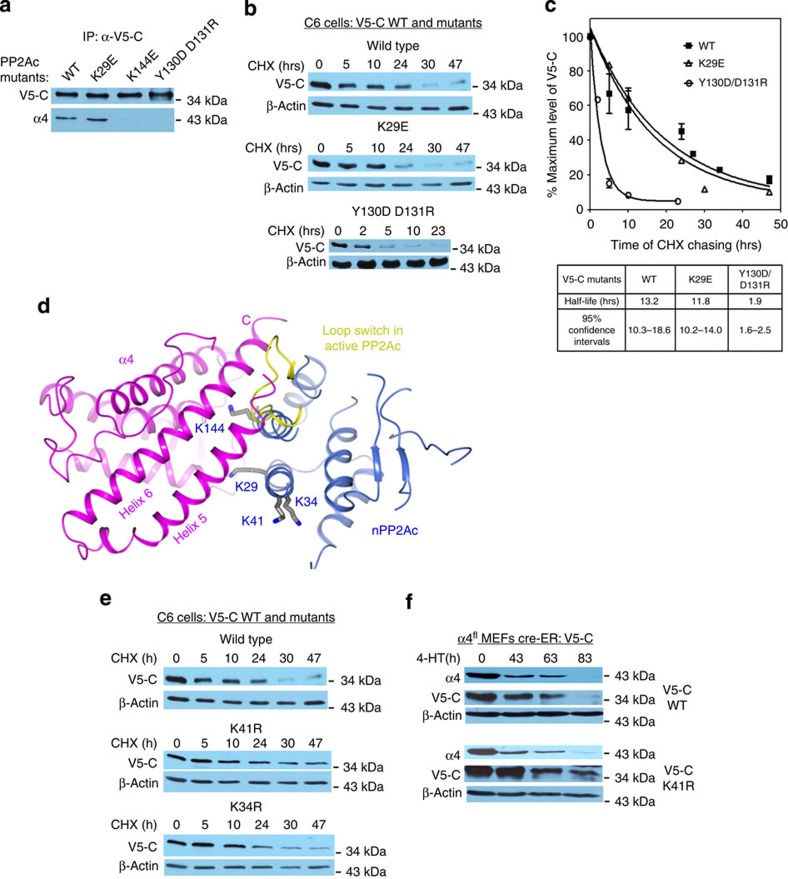
Figure 7. The PP2A–α4 interface is essential for the stability of total cellular PP2A.
(a) Co-IP determined the interaction between wild-type and mutant V5-C and α4 in C6 glioma cells. (b) Cycloheximide (CHX) chasing and western blot using an antibody that specifically detects V5-tag showed that the turnover rate of V5-C is accelerated by mutations (Y130D/D131R) that disrupt α4 interaction. K29E, a mutation located outside the interface, exhibited no effect. For panels a and b, experiments were repeated three times; representative results are shown. (c) Exponential decay simulation of the results of CHX chasing described in b determined the half-life of wild-type and mutant V5-C. The results shown are the average of three experiments and expressed as mean±s.e.m. (d) Structure of the nPP2Ac–α4 complex highlights PP2A lysine residues (grey) at or near the interface to α4. The loop switch in active PP2Ac is shown (yellow). α4 and nPP2Ac are shown in ribbon and coloured magenta and blue, respectively. (e) CHX chasing showed that the turnover rate of V5-C is reduced by mutation K41R near the interface to α4, but not by K34R. (f) K41R mutation attenuated the loss of V5-C upon α4 knockout.