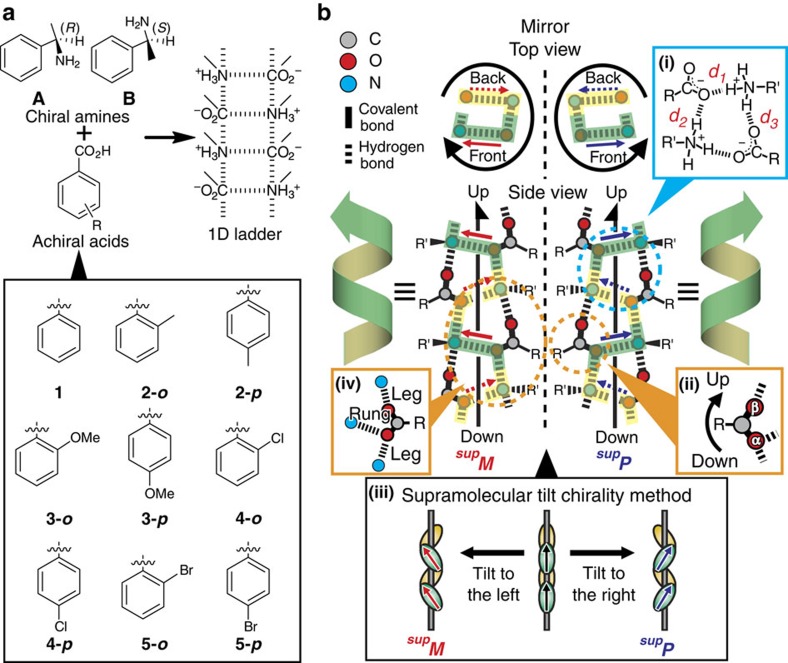
Figure 2. Helicity and handedness of one-dimensional ladder-type hydrogen-bonding networks.
(a) Primary ammonium carboxylates employed in this study. The organic salts are composed of chiral amine A or B with achiral benzoic acid derivatives (1–5). (b) Twofold helical hydrogen-bonding networks with repetition of deformed rings (inset (i)) based on discriminable O(α) and O(β) of carboxylate groups (inset (ii)). Right or left handedness are determined according to the supramolecular tilt chirality method (inset (iii)) and referred to as supP or supM, respectively. The rung N–O(α) portion (inset (iv)) is located on the front and back sides in the top views, which are indicated in green and yellow, respectively. The right or left tilt of the rung portions on the front sides defines the right- or left-handedness of the 21-helices (inset (iii)). The upward direction of the networks is defined along the direction of O(α) to O(β) on the leg portions (inset (ii)).