Abstract
Purpose
In IVF procedures, endometrial function is a limiting factor of the pregnancy rate and the aims of this study is to determine whether seminal plasma insemination at ovum pick-up in IVF has any effect on pregnancy rate.
Methods
We designed a single center, 2 parallel groups, randomized pilot study. All couples undergoing an IVF procedure in our clinic between January 2010 and December 2011 were considered for enrollment in the study. The patients who met the inclusion criteria were randomized into two groups by simple randomization: the seminal plasma group (SP group) and the reference group (noSP group). We applied seminal plasma into the cervix and vaginal vault at the time of the OPU in the patients of the SP group. The primary outcome was the clinical pregnancy rate in the SP group compared with the noSP group and the secondary outcome measure was the implantation rate.
Results
400 patients met the inclusion criteria and were randomized. 54 patients were excluded from the study mainly because they didn’t undergo the embryo transfer. Finally, 164 patients were included in the SP group and 182 patients in the noSP group and analyzed. We found a statistically non-significant increase in the pregnancy rate in the SP group (55.5 % versus 44 %, p = 0.09) and a statistically significant increase in the implantation rate (34.7 % versus 27.5 %, p = 0.026).
Conclusions
Our results support the idea that SP insemination may have the potential to increase pregnancy rate in IVF procedures but further studies must be carried out.
Keywords: IVF, Pregnancy rate, Endometrium, Implantation, Seminal plasma
Introduction
In IVF procedures, only 20 to 25 % of the transferred embryos lead to a pregnancy. Because this percentage cannot be further increased by the improvement of the embryo transfer technique and culture conditions or by an optimal selection of embryos, endometrial function and receptivity have been accepted to be the major limiting factors of IVF pregnancy rate. The endometrium is receptive to implantation in the mid luteal phase during the so called “implantation window” [14]. Its duration is determined by the sex hormones which regulate the expression of several cytokines [2]. Controlled ovarian stimulation (COH) during the IVF determines a different hormonal profile compared to the natural cycle and thus alters the endometrial function and receptivity. Several studies have revealed that exposure to seminal plasma (SP), the fluid component of ejaculate produced by seminal vesicles, may play a beneficial role in implantation. SP, which contains paternal alloantigens and high concentrations of cytokines, growth factors and prostaglandins, induce a state of active immune tolerance, essential for the embryo to be implanted [20]. Animals that become pregnant through artificial insemination or embryo transfer without being exposed to SP have substantially lower rates of implantation than those exposed to SP [7]. Excision of the seminal vesicle glands from males diminishes the tolerance-inducing effect of mating, while the vasectomy to remove sperms from the seminal fluid does not substantially impact the response [17].
These findings have led to the conclusion that the use of SP or its ingredients may stimulate endometrium in IVF cycles in order to improve implantation rates. Several studies have investigated the role of SP in implantation with controversial results, some reporting benefits [1,19,21], whereas others showing no effect [6,15].
The purpose of this study is to investigate whether the application of SP into the cervix and vagina at the time of the ovum pick-up has any influence on the pregnancy rates in IVF.
Material and methods
This was a single center, two groups, parallel-group, single blind, randomized study conducted at the IVF Department of the Polisano Clinic in Sibiu, Romania.
All patients undergoing an IVF procedure in our center between January 2010 and December 2011 were eligible to participate in the study. The inclusion criteria were: female age under 38 years, 0–3 IVF previous attempts. The exclusion criteria were: presence of male infection or leukocytospermia; presence of HIV infection, hepatitis B or C, syphilis in one or both partner; uterine diseases (congenital malformations, fibroids, and endometrial adhesions). Male infection was documented by the presence of leukocytospermia defined as leukocyte count of more than 1 million/ml ejaculate. Patients who met the eligibility criteria were divided in two groups: the SP group (who received SP insemination) and the noSP group (control group). To be considered for analysis the patients in both groups had to undergo an embryo transfer with top quality embryos. The ethical approval for the study was obtained from the institutional review board and the consent was obtained from patients.
The patients were randomly assigned to one group on the day of ovulation triggering by a nurse using a randomization list with a 1:1 ratio. The allocation sequence was concealed only to the patients.
The patients in SP group received seminal plasma insemination after the OPU. The SP was extracted from partner’s ejaculate by centrifugation of ejaculates at 700 × g for 15 min and then at 2,000 × g for 10 min. The supernatant was verified under the microscope to make sure that it is free of spermatozoa or other cells. 500 to 1,500 μl of supernatant, depending on the amount of sperm samples, were stored in an insemination catheter (Wallace, Smiths Medical North America). After the OPU, the catheter was introduced 1–2 cm into the cervical canal and 500 μl of SP was injected. The remaining SP was injected into the posterior vaginal vault. In both groups, COH was performed by using long-acting or antagonist protocols. The long protocol was used in women with a good ovarian reserve, endometriosis. The antagonist protocol was used in patients with a polycystic ovary syndrome, low ovarian reserve, previous low ovarian response or ovarian surgery. The down regulation was performed by administration of Diphereline 3.75 mg on the 21st day of the previous cycle. In the antagonist protocol, the patients received contraceptive pill for at least 18 days prior to the COH. In both protocols the patients received recombinant FSH (Gonal-F or Puregon) or highly purified hMG (Menopur); in the antagonist protocol, when the follicles reached 14 mm, the antagonist (Orgalutran) was added. The ovarian response was monitored by ultrasound scan. When at least 3 follicles reached 18 mm in diameter, the ovulation was triggered with 0.25 mg rhCG (Ovitrelle).
The ovum pick-up (OPU) was scheduled 36 h after the hCG administration and was performed transvaginally under general anesthesia.
The patients in both groups were instructed to abstain from sexual intercourse 3 days before and after the ovum pick-up until the time of the β-hCG result. Fertilization was achieved by standard IVF or ICSI, depending on the sperm count and morphology on the OPU day.
Intravaginal progesterone (Utrogestan 600 mg/day) was used as luteal support.
The embryos were cultured for 3 or 5 days. The embryo transfer was performed on day 3 or 5 under ultrasound guidance.
The embryo quality on day 3 was scored according to the following parameters: number of blastomeres, rate of fragmentation, multinucleation of the blastomeres, and early compaction. A top-quality embryo was considered to have at least eight cells on day 3, with ≤10 % fragmentation, regular size of the blastomeres and absence of multinucleation. The blastocyst quality was assessed according to the criteria of Gardner and Schoolcraft [8]. A top quality blastocyst was considered to be at least BL3, type A for the ICM and type A or B for the trophectoderm.
The primary outcome measure was clinical pregnancy at 4 weeks after the OPU, defined as the presence of the gestational sac or the presence of the embryonic pole. The secondary outcome measure was the implantation rate. Additional analysis was done on age, infertility cause, the COH protocol, the number of oocytes collected, the number of mature oocytes, the method of fertilization, the fertilization rate, the day of the embryo transfer, and the number of the transferred embryos. Because this was a pilot study we didn’t calculate the sample size. Continuous variables were compared using Student’s t-test and Levene’s test. Categorical variables were compared using the chi squared test. The statistical significance was accepted when p ≤ 0.05. The statistical analysis was performed with SPSS 17.0 software (SPSS Inc., Chicago, IL, USA)
Results
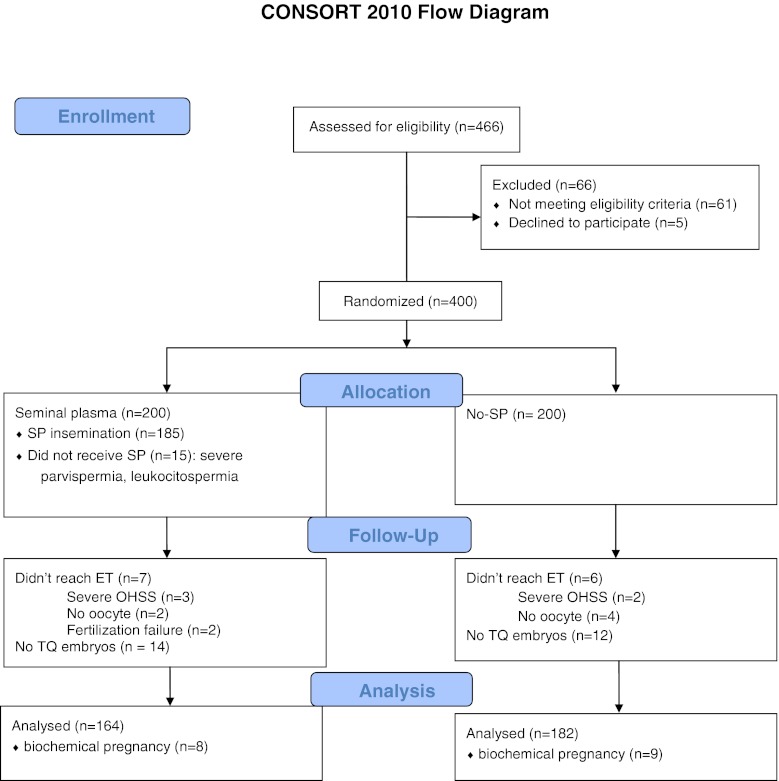
466 patients who underwent an IVF procedure in our center were eligible to participate in the study. 54 patients were excluded because they didn’t meet the inclusion criteria or they met one or more exclusion criteria. 12 patients were excluded because they were poor responders and didn’t reach the OPU. 400 patients were randomized into two groups on the day of ovulation triggering by a randomization list. 36 patients in the SP group and 18 patients in the noSP group were excluded because they didn’t have top quality embryos or didn’t undergo the embryo transfer. Finally, 164 patients in SP group and 182 patients in noSP group were analyzed (CONSORT Flow chart).
CONSORT 2010 Flow Diagram
As shown in Table 1, no significant differences were recorded between the two groups in term of mean age, cause of infertility, stimulation protocols, fertilization type (IVF or ICSI), mean number of embryos transferred and day of the embryo transfer.
Table 1.
Baseline characteristics of both groups
| Baseline characteristics | SP group (n = 164) | No-SP group (n = 182) | P value |
|---|---|---|---|
| Mean age (years) | 33.1 (25–37) | 33.9 (23–37) | NS |
| Duration of infertility (years) | 3.8 | 4.1 | NS |
| No of previous IVF attempts | 1 (0–3) | 1(0–3) | NS |
| Cause of infertility n(%) | NS | ||
| Tubal factor | 53(32.3) | 70(38.5) | |
| Male factor | 62(37.8) | 64(35.2) | |
| Endometriosis | 2(1.2) | 6(3.3) | |
| PCOS | 11(6.7) | 5(2.7) | |
| Unexplained | 36(22) | 37(20.3) | |
| Stimulation protocol n(%) | NS | ||
| Long | 93(56.7) | 99(54.4) | |
| Antagonist | 71(43.3) | 83(45.6) | |
| Method of fertilization n(%) | NS | ||
| Standard IVF | 58(35.4) | 81(44.5) | |
| ICSI | 106(64.6) | 101(55.5) | |
| No of transferred embryos (median) | 2.27 (1–3) | 2.31 (1–3) | NS |
| Day of embryo transfer n(%) | NS | ||
| Day 3 | 68 (49.5) | 89 (48.9) | |
| Day 5 | 96 (50.5) | 93 (51.1) | |
The results are expressed as mean values. The statistical analysis was performed using the chi square test for the day of the transfer, the fertilization method and the number of transferred embryos
Also, no significant differences were found regarding the number of oocytes collected, mature oocytes and the fertilization rate (Table 2). Interestingly, we found that in the noSP group the number of top quality embryos was higher than in the SP group.
Table 2.
The embryological data in both groups
| Embryological data | SP group | noSP group | P value |
|---|---|---|---|
| Number of collected oocytes | 14.5 ± 7.7 | 13.3 ± 7 | NS |
| Number of mature oocytes | 11.6 ± 6.3 | 10.6 ± 5.5 | NS |
| Fertilization rate % (*) | 76.7 (1,437/1,897) | 72 (1,395/1,938) | NS |
| Top quality embryo (n) | 3.15 ± 1.9 | 3.74 ± 2.35 | 0.01 |
Values are expressed as means and standard deviation. The statistical analysis was performed by using the t-test
*number of embryos obtained/number of mature oocyte collected
We found a non-significant increase in the clinical pregnancy rate in the SP group compared with the noSP group (55.5 % versus 44 %, p = 0.09). Also, using the one-tailed t-test we found a significant increase in implantation rate in SP group (34.7 % versus 27.5 %, p = 0.026) (Table 3). Calculating the sample size for these results to become statistically significant we found that 313 patients are needed on each group.
Table 3.
The outcome parameters in both groups
| Outcome measures | SP group | noSP group | P value |
|---|---|---|---|
| Pregnancy n (%) | clinical | 91 (55.5 %) | 80 (44 %) |
| negative | 65 (39.6 %) | 93 (51.1 %) | |
| biochemical | 8 (4.9 %) | 9 (4.9 %) | |
| Clinical pregnancy (n) | Single | 61 | 45 |
| Twins | 28 | 33 | |
| triplets | 2 | 2 | |
| Pregnancy rate (%) | 55.5 % (91/164) | 44 % (80/182) | 0.09 |
| Implantation rate % (*) | 34.7 (123/372) | 27.5 (117/425) | 0.026** |
*number of embryos with heart beat on ultrasound/number of embryos transferred
**one-tailed t-test
Discussion
The results of this study support the idea that SP insemination during the OPU in IVF cycles improves the pregnancy rate. Our results reach statistical significance only in the implantation rate (34.7 % in the SP group versus 27.5 % in the noSP group, p = 0.026). Unfortunately, many patients were lost to follow-up, so we cannot draw a conclusion regarding the effect of the SP insemination on the live birth’s rate.
Several studies have investigated the role of SP in implantation. Qasim found that application of SP in vagina during intrauterine insemination does not improve the pregnancy rate [15]. Fishel found no difference in the pregnancy and miscarriage rates between 2 groups with or without SP (32 % versus 33 % and 21 % versus 17 %) [6]. Bellinge deposited semen in the vagina of patients undergoing IVF at the time of the oocyte fertilization and found an implantation rate of 53 %, compared with 23 % in the control group [1]. Tremellen randomized 600 patients who either abstained from or engaged in vaginal intercourse around the day of the embryo transfer and found a significantly higher number of viable embryos at 6–8 weeks of pregnancy in the second group although pregnancy rates were similar in both groups [19]. Coulam and Stern performed a placebo-controlled clinical trial, depositing into the vagina capsules containing SP or placebo. They described an implantation rate of 80 % in the SP group, compared with 67 % in the placebo group [4]. Von Wolff applied thawed SP at the time of OPU during IVF-ICSI cycles and found a non-significant increase of the pregnancy rate (37.3 % versus 25.7 %) [21].
Therefore, as the designs of these studies are heterogeneous, the results are difficult to compare. Our study reaches statistical significance in implantation rates and the results are dependent on the SP insemination; all the other factors affecting the pregnancy rate were similar in both groups. One interesting fact is that in the noSP group the number of top quality embryos obtained was higher than in the SP group with no evident explanation.
The SP may influence the endometrium through three possible mechanisms. First, some of the SP may reach the uterine cavity through the cervical canal, with direct stimulatory effect on the endometrium [13]. Second, the concept of vascular countercurrent transfer between the vagina and the uterus provides a very interesting model to explain the stimulatory effect of SP on the endometrium, without ascension through the cervical canal [5]. Some constituents of SP may reach the endometrium through the vascular system by the so-called first-pass effect. Cicinelli found a 10–20 fold increase in the level of progesterone in endometrial cells after the vaginal administration compared to the parenteral administration at the same peripheral plasma levels [3]. Third, the SP may provide paternal alloantigens, modulating the female immune system to better accept the embryo. This tolerance is though to be mediated at least in part by regulatory T (Treg) cells, a subpopulation of T cells which are potent suppressors of inflammatory type 1 (cell-mediated) immune responses [9]. This concept is supported by experiments in mice. Johansson demonstrated that activation and expansion of the female lymphocyte populations after mating were triggered by constituents of SP derived from seminal vesicle glands [11]. SP determines an inflammation-like response in the female reproductive tract associated with recruitment of dendritic cells into the endometrium [18]. These cells process the male antigens from SP and activate proliferation and activation of regulatory T cells [11] which promote tolerance of paternal alloantigens at the time of the embryo implantation [17]. The SP also contains prostaglandin E and TGF-β, capable of initiating tolerance towards foreign antigens [12]. These cytokines are likely to be important in skewing the T-cells towards the Treg cell subpopulation [16]. Exposure to SP on the day of the OPU may trigger the immune tolerance to the paternal antigens and thus improve the embryo implantation.
Furthermore, the in vitro stimulation of endometrial epithelial cells with SP up-regulates several endometrial factors, such as the leukemia inhibitory factor (LIF) and IL-6, which are thought to play an important role in human implantation and the early stages of pregnancy [10]. The great number of active constituents of the SP supports the assumption that the priming of the endometrium by the SP is dependent on several factors rather than on a single stimulatory agent.
In conclusion, our results support the hypothesis that SP has potential to increase pregnancy rate in IVF procedures, but further, more comprehensive studies are needed in the future.
Footnotes
Capsule
Seminal plasma insemination in IVF.
References
- 1.Bellinge BS, Copeland CM, Thomas TD, Mazzucchelli RE, O’Neil G, Cohen MJ. The influence of patient insemination on the implantation rate in an in vitro fertilization and embryo transfer program. Fertil Steril. 1986;46:25–26. doi: 10.1016/s0015-0282(16)49521-x. [DOI] [PubMed] [Google Scholar]
- 2.Boomsma CM, Kavelaars A, Eijkemans MJC, Lentjes EG, Fauser BCJM, Heijnen CJ, et al. Endometrial secretion analysis identifies a cytokine profile predictive of pregnancy in IVF. Hum Reprod. 2009;24(6):1427–1435. doi: 10.1093/humrep/dep011. [DOI] [PubMed] [Google Scholar]
- 3.Cicinelli E, de Ziegler D. Transvaginal progesterone: evidence for a new functional “portal system” flowing from the vagina to the uterus. Hum Reprod Updat. 1999;5:365–372. doi: 10.1093/humupd/5.4.365. [DOI] [PubMed] [Google Scholar]
- 4.Coulam CB, Stern JJ. Effect of seminal plasma on implantation rates. Early Pregnancy. 1995;1:33–36. [PubMed] [Google Scholar]
- 5.Einer-Jensen N, Hunter R. Counter-current transfer in reproductive biology. Reproduction. 2005;129:9–18. doi: 10.1530/rep.1.00278. [DOI] [PubMed] [Google Scholar]
- 6.Fishel S, Webster J, Jackson P, Faratian B. Evaluation of high vaginal insemination at oocyte recovery in patients undergoing in vitro fertilization. Fertil Steril. 1989;51:135–138. doi: 10.1016/s0015-0282(16)60442-9. [DOI] [PubMed] [Google Scholar]
- 7.Flowers WL, Esbenshade KL. Optimizing management of natural and artificial matings in swine. J Reprod Fertil Suppl. 1993;48:217–228. [PubMed] [Google Scholar]
- 8.Gardner DK, Schoolcraft WB. Towards reproductive certainty. In: Jansen R, Mortimer D, editors. Fertility and genetics beyond. Carnforth: Parthenon Publishing; 1999. pp. 378–388. [Google Scholar]
- 9.Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod. 2009;15:517–535. doi: 10.1093/humupd/dmp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutsche S, von Wolff M, Strowitzki T, Thaler CJ. Seminal plasma induces mRNA expression of IL-1beta, IL-6 and LIF in endometrial epithelial cells in vitro. Mol Hum Reprod. 2003;9:785–791. doi: 10.1093/molehr/gag095. [DOI] [PubMed] [Google Scholar]
- 11.Johansson M, Bromfield JJ, Jasper MJ, Robertson SA. Semen activates the female immune response during early pregnancy in mice. Immunology. 2004;112:290–300. doi: 10.1111/j.1365-2567.2004.01876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly RW, Carr GC, Critchley HO. A cytokine switch induced by human seminal plasma: an immunemodulation with implications for sexually transmitted disease. Hum Reprod. 1997;12:677–681. doi: 10.1093/humrep/12.4.677. [DOI] [PubMed] [Google Scholar]
- 13.Kolibianakis EM, Bourgain C, Platteau P, Albano C, Van Steirteghem AC, Devroey P. Abnormal endometrial development occurs during the luteal phase of nonsupplemented donor cycles treated with recombinant follicle-stimulating hormone and gonadotropin-releasing hormone antagonists. Fertil Steril. 2003;80:464–466. doi: 10.1016/S0015-0282(03)00663-0. [DOI] [PubMed] [Google Scholar]
- 14.Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A. 2003;100:2963–2968. doi: 10.1073/pnas.0530162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qasim SM, Trias A, Karacan M, Shelden R, Kemmann E. Does the absence or presence of seminal fluid matter in patients undergoing ovulation induction with intrauterine insemination? Hum Reprod. 1996;11(5):1008–1010. doi: 10.1093/oxfordjournals.humrep.a019285. [DOI] [PubMed] [Google Scholar]
- 16.Robertson SA, Ingman WV, O’Leary S, Sharkey DJ, Tremellen KP. Transforming growth factor beta—a mediator of immune deviation in seminal plasma. J Reprod Immunol. 2002;57:109–128. doi: 10.1016/S0165-0378(02)00015-3. [DOI] [PubMed] [Google Scholar]
- 17.Robertson SA, Guerin LR, Moldenhauer M, Hayball JD. Activating T regulatory cells for tolerance in early pregnancy-the contribution of seminal fluid. J Reprod Immunol. 2009;83:109–116. doi: 10.1016/j.jri.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Sharkey DJ, Macpherson AM, Tremellen KP, Robertson SA. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod. 2007;13:491–501. doi: 10.1093/molehr/gam028. [DOI] [PubMed] [Google Scholar]
- 19.Tremellen KP, Valbuena D, Landeras J, et al. The effect of intercourse on pregnancy rates during assisted human reproduction. Hum Reprod. 2000;15:2653–2658. doi: 10.1093/humrep/15.12.2653. [DOI] [PubMed] [Google Scholar]
- 20.Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7:241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 21.Von Wolff M, Rosner S, Thone C, Pinheiro RM, Jauckus J, Bruckner T, et al. Intravaginal and intracervical application of seminal plasma in in vitro fertilization or intracytoplasmic sperm injection treatment cycles—a double-blind, placebo-controlled, randomized pilot study. Fertil Steril. 2009;91(1):167–172. doi: 10.1016/j.fertnstert.2007.11.036. [DOI] [PubMed] [Google Scholar]