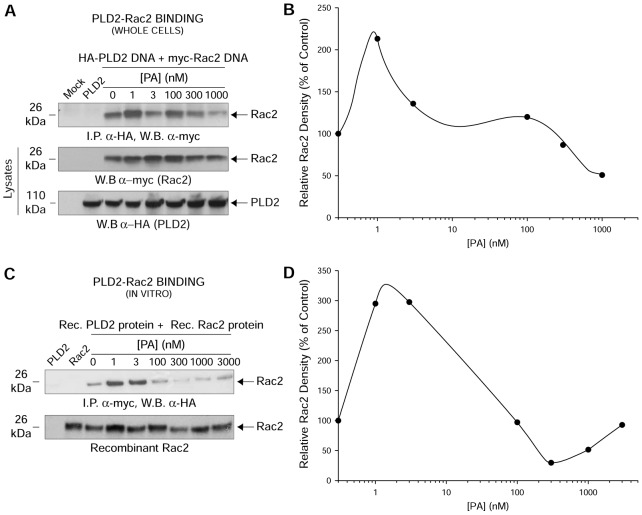
Fig. 2.
PA interferes with protein–protein interaction between PLD2 and Rac2. (A) In vivo PLD2–Rac2 binding in the absence or presence of increasing PA. COS-7 cells were co-transfected with constant myc-tagged Rac2 and constant HA-tagged PLD2-WT. HA-tagged PLD2-WT was immunoprecipitated using mouse anti-HA-agarose followed by subsequent western blotting (W.B.) using rabbit anti-myc antibody specific for the myc-tagged Rac2. (B,D) Densitometry line graphs of in vivo and in vitro immunoprecipitations shown in A and C, respectively. Representative total cellular Rac2 and PLD2 (20% of total cellular protein) or recombinant Rac2 equal loading controls are shown in the last two panels of A or bottom panel of C, respectively. (C) In vitro PLD2–Rac2 binding in the absence or presence of increasing PA. Purified, baculoviral myc-tagged Rac2 and HA-tagged PLD2-WT proteins were used for in vitro binding assays. Similar to A, myc-tagged PLD2-WT was immunoprecipitated using mouse anti-myc-agarose followed by subsequent western blotting using rabbit anti-HA antibody specific for the HA-tagged Rac2. Results shown are representative of three independent experiments performed in duplicate.