Fig. 3.
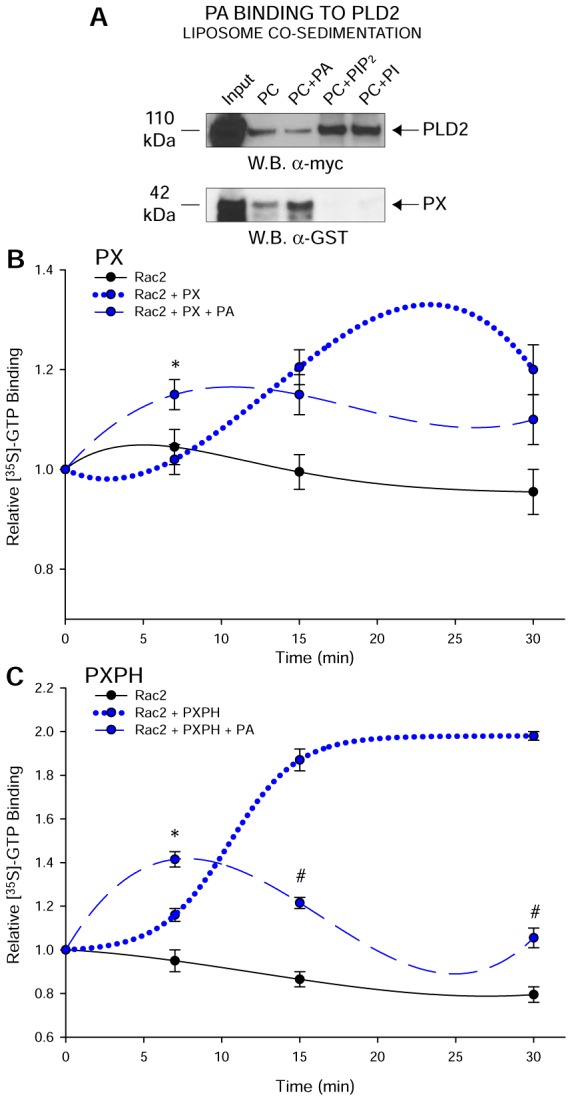
PA binds PLD2 at the PX domain and negatively regulates Rac2 binding. (A) Liposome co-sedimentation assays using purified, recombinant full-length PLD2 or GST–PX (purified). Liposomes composed of phosphatidylcholine (PC), both PC and PA, both PC and PI(3)P or both PC and PI(4,5)P2 (weight ratio 6∶4) were incubated with purified full length PLD2 or GST–PX followed by SDS and western blot (W.B.) analyses. Full length PLD2 interacts with all the four lipids including PA. PX domain binds to PA preferentially but not to PI or PIP2. Lane 1 of all the panels shows protein input in the liposome co-sedimentation assay. (B,C) Negative effect of PA on [35S]-GTP binding of Rac2 mediated by PX (B) and PXPH (C) respectively. GTP binding assays were performed in the absence or presence of 300 nM PA. Triplicate results are means±s.e.m. and are expressed in terms of relative [35S]-GTPγS binding. Curves in B and C were best fit by a polynomial cubic equation. *denotes significant differences (P<0.005) above controls; #denotes significant differences (P<0.005) below controls.
