Abstract
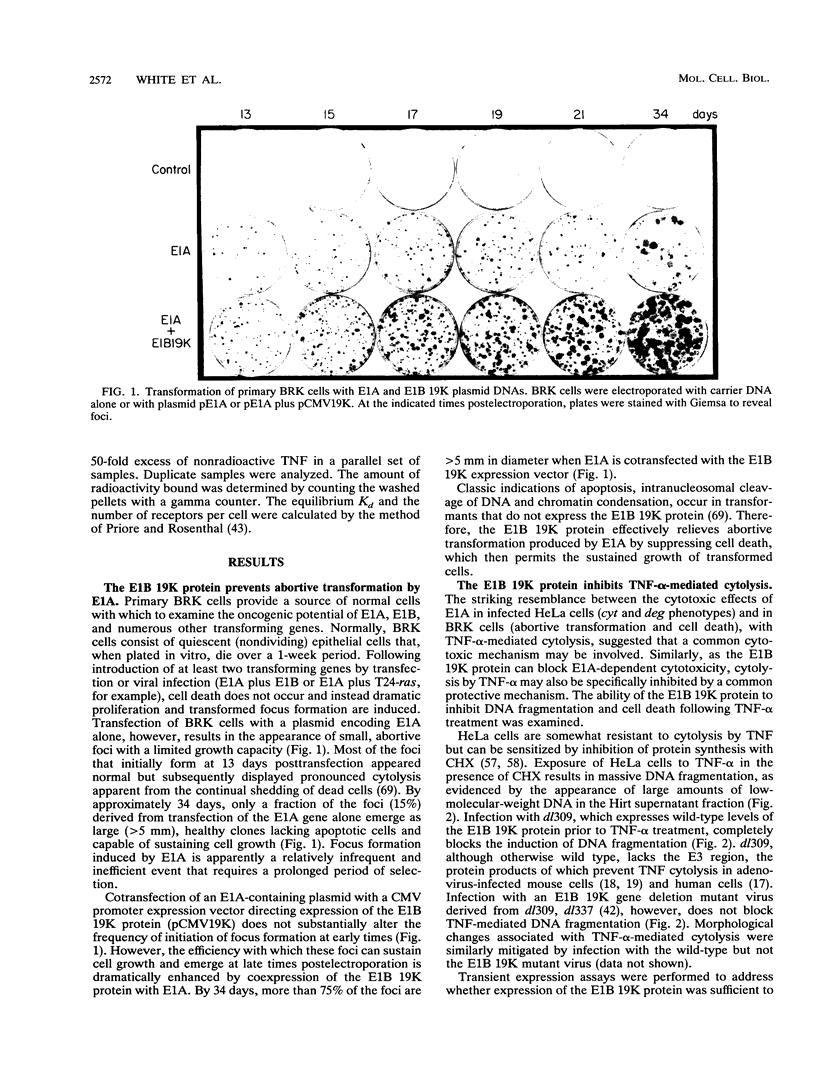
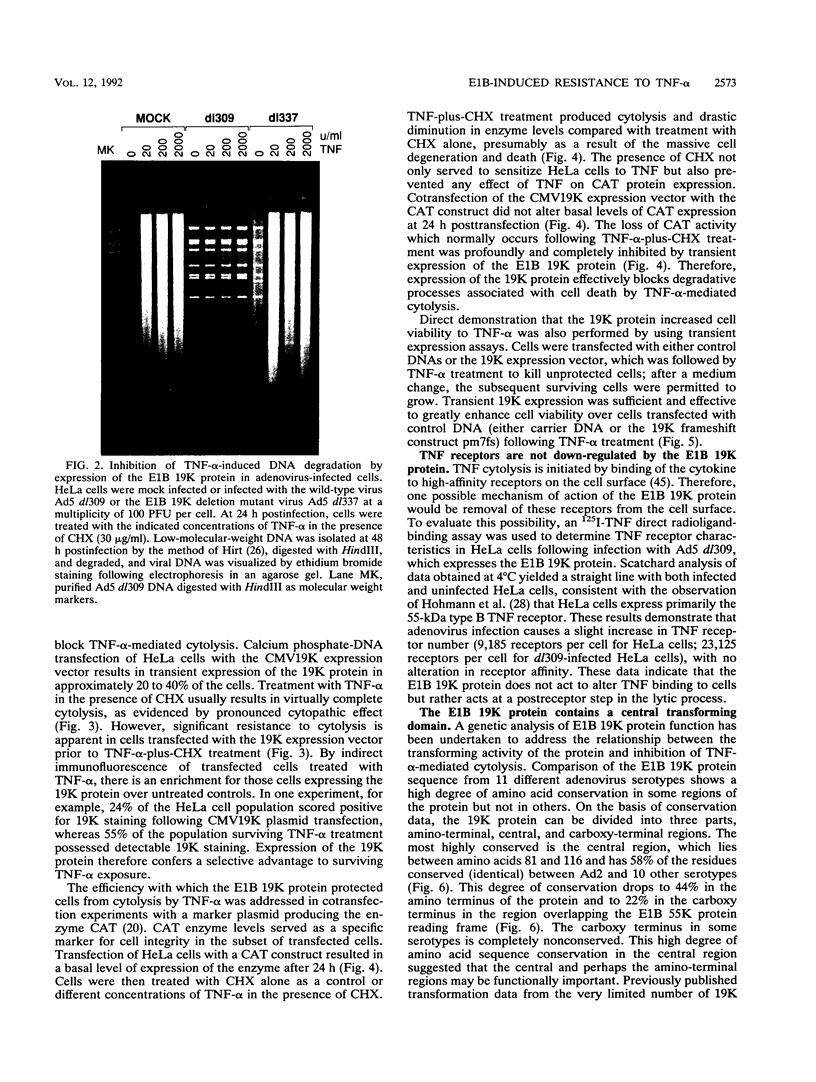
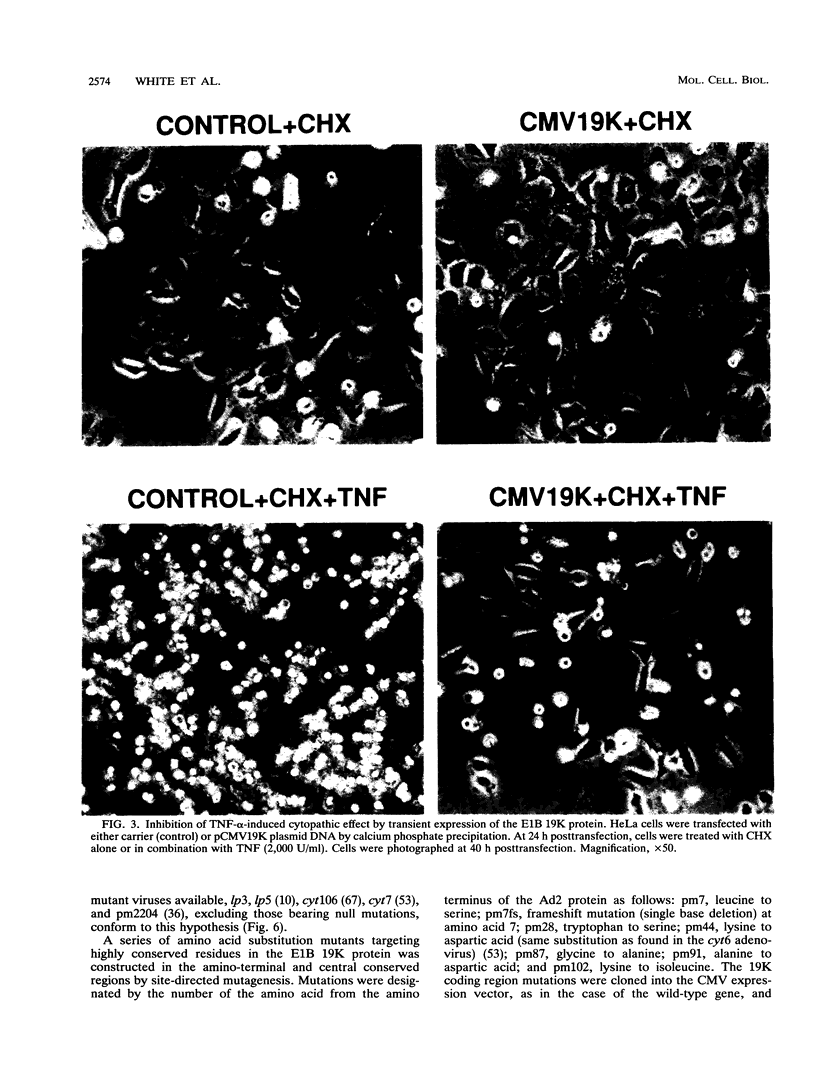
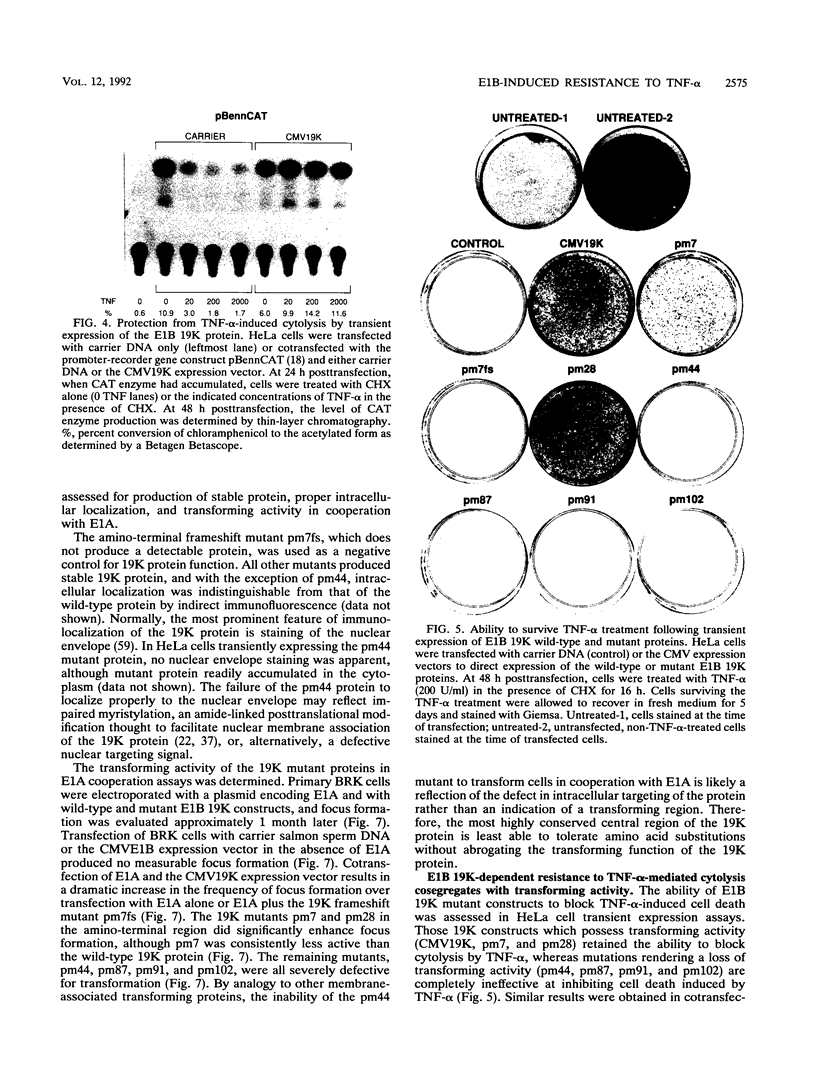
The adenovirus E1A and E1B proteins are required for transformation of primary rodent cells. When expressed in the absence of the 19,000-dalton (19K) E1B protein, however, the E1A proteins are acutely cytotoxic and induce host cell chromosomal DNA fragmentation and cytolysis, analogous to cells undergoing programmed cell death (apoptosis). E1A alone can efficiently initiate the formation of foci which subsequently undergo abortive transformation whereby stimulation of cell growth is counteracted by continual cell death. Cell lines with an immortalized growth potential eventually arise with low frequency. Coexpression of the E1B 19K protein with E1A is sufficient to overcome abortive transformation to produce high-frequency transformation. Like E1A, the tumoricidal cytokine tumor necrosis factor alpha (TNF-alpha) evokes a programmed cell death response in many tumor cell lines by inducing DNA fragmentation and cytolysis. Expression of the E1B 19K protein by viral infection, by transient expression, or in transformed cells completely and specifically blocks this TNF-alpha-induced DNA fragmentation and cell death. Cosegregation of 19K protein transforming activity with protection from TNF-alpha-mediated cytolysis demonstrates that both activities are likely the consequence of the same function of the protein. Therefore, we propose that by suppressing an intrinsic cell death mechanism activated by TNF-alpha or E1A, the E1B 19K protein enhances the transforming activity of E1A and enables adenovirus to evade TNF-alpha-dependent immune surveillance.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames R. S., Holskin B., Mitcho M., Shalloway D., Chen M. J. Induction of sensitivity to the cytotoxic action of tumor necrosis factor alpha by adenovirus E1A is independent of transformation and transcriptional activation. J Virol. 1990 Sep;64(9):4115–4122. doi: 10.1128/jvi.64.9.4115-4122.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. D., Berk A. J. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1987 Jan;156(1):107–121. doi: 10.1016/0042-6822(87)90441-7. [DOI] [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Bernards R., de Leeuw M. G., Houweling A., van der Eb A. J. Role of the adenovirus early region 1B tumor antigens in transformation and lytic infection. Virology. 1986 Apr 15;150(1):126–139. doi: 10.1016/0042-6822(86)90272-2. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Molecular themes in oncogenesis. Cell. 1991 Jan 25;64(2):235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Braithwaite A. W., Nelson C. C., Bellett A. J. E1a revisited: the case for multiple cooperative trans-activation domains. New Biol. 1991 Jan;3(1):18–26. [PubMed] [Google Scholar]
- Branton P. E., Bayley S. T., Graham F. L. Transformation by human adenoviruses. Biochim Biophys Acta. 1985;780(1):67–94. doi: 10.1016/0304-419x(84)90007-6. [DOI] [PubMed] [Google Scholar]
- Chen M. J., Holskin B., Strickler J., Gorniak J., Clark M. A., Johnson P. J., Mitcho M., Shalloway D. Induction by E1A oncogene expression of cellular susceptibility to lysis by TNF. Nature. 1987 Dec 10;330(6148):581–583. doi: 10.1038/330581a0. [DOI] [PubMed] [Google Scholar]
- Chinnadurai G. Adenovirus 2 Ip+ locus codes for a 19 kd tumor antigen that plays an essential role in cell transformation. Cell. 1983 Jul;33(3):759–766. doi: 10.1016/0092-8674(83)90018-1. [DOI] [PubMed] [Google Scholar]
- Cook J. L., May D. L., Wilson B. A., Holskin B., Chen M. J., Shalloway D., Walker T. A. Role of tumor necrosis factor-alpha in E1A oncogene-induced susceptibility of neoplastic cells to lysis by natural killer cells and activated macrophages. J Immunol. 1989 Jun 15;142(12):4527–4534. [PubMed] [Google Scholar]
- Dealtry G. B., Naylor M. S., Fiers W., Balkwill F. R. DNA fragmentation and cytotoxicity caused by tumor necrosis factor is enhanced by interferon-gamma. Eur J Immunol. 1987 May;17(5):689–693. doi: 10.1002/eji.1830170517. [DOI] [PubMed] [Google Scholar]
- Duerksen-Hughes P. J., Hermiston T. W., Wold W. S., Gooding L. R. The amino-terminal portion of CD1 of the adenovirus E1A proteins is required to induce susceptibility to tumor necrosis factor cytolysis in adenovirus-infected mouse cells. J Virol. 1991 Mar;65(3):1236–1244. doi: 10.1128/jvi.65.3.1236-1244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerksen-Hughes P., Wold W. S., Gooding L. R. Adenovirus E1A renders infected cells sensitive to cytolysis by tumor necrosis factor. J Immunol. 1989 Dec 15;143(12):4193–4200. [PubMed] [Google Scholar]
- Flint S. J. Cellular transformation by adenoviruses. Pharmacol Ther. 1984;26(1):59–88. doi: 10.1016/0163-7258(84)90051-2. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Phelps W., Feigenbaum L., Ostrove J. M., Adachi A., Howley P. M., Khoury G., Ginsberg H. S., Martin M. A. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9759–9763. doi: 10.1073/pnas.83.24.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R., Aquino L., Duerksen-Hughes P. J., Day D., Horton T. M., Yei S. P., Wold W. S. The E1B 19,000-molecular-weight protein of group C adenoviruses prevents tumor necrosis factor cytolysis of human cells but not of mouse cells. J Virol. 1991 Jun;65(6):3083–3094. doi: 10.1128/jvi.65.6.3083-3094.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R., Elmore L. W., Tollefson A. E., Brady H. A., Wold W. S. A 14,700 MW protein from the E3 region of adenovirus inhibits cytolysis by tumor necrosis factor. Cell. 1988 May 6;53(3):341–346. doi: 10.1016/0092-8674(88)90154-7. [DOI] [PubMed] [Google Scholar]
- Gooding L. R., Ranheim T. S., Tollefson A. E., Aquino L., Duerksen-Hughes P., Horton T. M., Wold W. S. The 10,400- and 14,500-dalton proteins encoded by region E3 of adenovirus function together to protect many but not all mouse cell lines against lysis by tumor necrosis factor. J Virol. 1991 Aug;65(8):4114–4123. doi: 10.1128/jvi.65.8.4114-4123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Roberts C., Gallimore P. H. Acylation of adenovirus type 12 early region 1b 18-kDa protein. Further evidence for its localisation in the cell membrane. FEBS Lett. 1985 Feb 25;181(2):229–235. doi: 10.1016/0014-5793(85)80265-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Ishii A., Yonehara S. The E1b oncogene of adenovirus confers cellular resistance to cytotoxicity of tumor necrosis factor and monoclonal anti-Fas antibody. Int Immunol. 1991 Apr;3(4):343–351. doi: 10.1093/intimm/3.4.343. [DOI] [PubMed] [Google Scholar]
- Henderson S., Rowe M., Gregory C., Croom-Carter D., Wang F., Longnecker R., Kieff E., Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991 Jun 28;65(7):1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- Herrmann C. H., Dery C. V., Mathews M. B. Transactivation of host and viral genes by the adenovirus E1B 19K tumor antigen. Oncogene. 1987;2(1):25–35. [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Hohmann H. P., Remy R., Brockhaus M., van Loon A. P. Two different cell types have different major receptors for human tumor necrosis factor (TNF alpha). J Biol Chem. 1989 Sep 5;264(25):14927–14934. [PubMed] [Google Scholar]
- Houweling A., van den Elsen P. J., van der Eb A. J. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980 Sep;105(2):537–550. doi: 10.1016/0042-6822(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L., Ferguson B., Rosenberg M., Baserga R. Induction of cellular DNA synthesis by purified adenovirus E1A proteins. Virology. 1986 Jul 15;152(1):1–10. doi: 10.1016/0042-6822(86)90366-1. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laster S. M., Wood J. G., Gooding L. R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988 Oct 15;141(8):2629–2634. [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- McGlade C. J., Tremblay M. L., Branton P. E. Mapping of a phosphorylation site in the 176R (19 kDa) early region 1B protein of human adenovirus type 5. Virology. 1989 Jan;168(1):119–127. doi: 10.1016/0042-6822(89)90410-8. [DOI] [PubMed] [Google Scholar]
- McGlade C. J., Tremblay M. L., Yee S. P., Ross R., Branton P. E. Acylation of the 176R (19-kilodalton) early region 1B protein of human adenovirus type 5. J Virol. 1987 Oct;61(10):3227–3234. doi: 10.1128/jvi.61.10.3227-3234.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLorie W., McGlade C. J., Takayesu D., Branton P. E. Individual adenovirus E1B proteins induce transformation independently but by additive pathways. J Gen Virol. 1991 Jun;72(Pt 6):1467–1471. doi: 10.1099/0022-1317-72-6-1467. [DOI] [PubMed] [Google Scholar]
- Mestan J., Digel W., Mittnacht S., Hillen H., Blohm D., Möller A., Jacobsen H., Kirchner H. Antiviral effects of recombinant tumour necrosis factor in vitro. 1986 Oct 30-Nov 5Nature. 323(6091):816–819. doi: 10.1038/323816a0. [DOI] [PubMed] [Google Scholar]
- Moran E., Mathews M. B. Multiple functional domains in the adenovirus E1A gene. Cell. 1987 Jan 30;48(2):177–178. doi: 10.1016/0092-8674(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Natarajan V. Adenovirus-2 E1a and E1b gene products regulate enhancer mediated transcription. Nucleic Acids Res. 1986 Dec 9;14(23):9445–9456. doi: 10.1093/nar/14.23.9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilder S., Logan J., Shenk T. Deletion of the gene encoding the adenovirus 5 early region 1b 21,000-molecular-weight polypeptide leads to degradation of viral and host cell DNA. J Virol. 1984 Nov;52(2):664–671. doi: 10.1128/jvi.52.2.664-671.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priore R. L., Rosenthal H. E. A statistical method for the estimation of binding parameters in a complex system. Anal Biochem. 1976 Jan;70(1):231–240. doi: 10.1016/s0003-2697(76)80063-2. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri P., Bagchi S., Devoto S. H., Kraus V. B., Moran E., Nevins J. R. Domains of the adenovirus E1A protein required for oncogenic activity are also required for dissociation of E2F transcription factor complexes. Genes Dev. 1991 Jul;5(7):1200–1211. doi: 10.1101/gad.5.7.1200. [DOI] [PubMed] [Google Scholar]
- Rubin B. Y., Anderson S. L., Sullivan S. A., Williamson B. D., Carswell E. A., Old L. J. High affinity binding of 125I-labeled human tumor necrosis factor (LuKII) to specific cell surface receptors. J Exp Med. 1985 Sep 1;162(3):1099–1104. doi: 10.1084/jem.162.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Sarnow P., Ho Y. S., Williams J., Levine A. J. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982 Feb;28(2):387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- Shenk T., Flint J. Transcriptional and transforming activities of the adenovirus E1A proteins. Adv Cancer Res. 1991;57:47–85. doi: 10.1016/s0065-230x(08)60995-1. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Kato H., Kawai S. Tandemly repeated hexamer sequences within the beta interferon promoter can function as an inducible regulatory element in activation by the adenovirus E1B 19-kilodalton protein. J Virol. 1990 Jun;64(6):3063–3068. doi: 10.1128/jvi.64.6.3063-3068.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabel S., Argos P., Philipson L. The release of growth arrest by microinjection of adenovirus E1A DNA. EMBO J. 1985 Sep;4(9):2329–2336. doi: 10.1002/j.1460-2075.1985.tb03934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian T., Chinnadurai G. Separation of the functions controlled by adenovirus 2 lp+ locus. Virology. 1986 Apr 30;150(2):381–389. doi: 10.1016/0042-6822(86)90303-x. [DOI] [PubMed] [Google Scholar]
- Subramanian T., Kuppuswamy M., Gysbers J., Mak S., Chinnadurai G. 19-kDa tumor antigen coded by early region E1b of adenovirus 2 is required for efficient synthesis and for protection of viral DNA. J Biol Chem. 1984 Oct 10;259(19):11777–11783. [PubMed] [Google Scholar]
- Takemori N., Cladaras C., Bhat B., Conley A. J., Wold W. S. cyt gene of adenoviruses 2 and 5 is an oncogene for transforming function in early region E1B and encodes the E1B 19,000-molecular-weight polypeptide. J Virol. 1984 Dec;52(3):793–805. doi: 10.1128/jvi.52.3.793-805.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori N., Riggs J. L., Aldrich C. Genetic studies with tumorigenic adenoviruses. I. Isolation of cytocidal (cyt) mutants of adenovirus type 12. Virology. 1968 Dec;36(4):575–586. doi: 10.1016/0042-6822(68)90189-x. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Finger L. R., Yunis J., Nowell P. C., Croce C. M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984 Nov 30;226(4678):1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B., Timmers H. T., Pronk G. J., van Roy F., Van der Eb A. J., Fiers W. Modulation of cellular susceptibility to the cytotoxic/cytostatic action of tumor necrosis factor by adenovirus E1 gene expression is cell type-dependent. Virology. 1990 Jun;176(2):362–368. doi: 10.1016/0042-6822(90)90006-d. [DOI] [PubMed] [Google Scholar]
- Wallach D. Cytotoxins (tumour necrosis factor, lymphotoxin and others): molecular and functional characteristics and interactions with interferons. Interferon. 1986;7:89–124. [PubMed] [Google Scholar]
- Wallach D. Preparations of lymphotoxin induce resistance to their own cytotoxic effect. J Immunol. 1984 May;132(5):2464–2469. [PubMed] [Google Scholar]
- White E., Cipriani R. Role of adenovirus E1B proteins in transformation: altered organization of intermediate filaments in transformed cells that express the 19-kilodalton protein. Mol Cell Biol. 1990 Jan;10(1):120–130. doi: 10.1128/mcb.10.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Cipriani R., Sabbatini P., Denton A. Adenovirus E1B 19-kilodalton protein overcomes the cytotoxicity of E1A proteins. J Virol. 1991 Jun;65(6):2968–2978. doi: 10.1128/jvi.65.6.2968-2978.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Cipriani R. Specific disruption of intermediate filaments and the nuclear lamina by the 19-kDa product of the adenovirus E1B oncogene. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9886–9890. doi: 10.1073/pnas.86.24.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Denton A., Stillman B. Role of the adenovirus E1B 19,000-dalton tumor antigen in regulating early gene expression. J Virol. 1988 Sep;62(9):3445–3454. doi: 10.1128/jvi.62.9.3445-3454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Faha B., Stillman B. Regulation of adenovirus gene expression in human WI38 cells by an E1B-encoded tumor antigen. Mol Cell Biol. 1986 Nov;6(11):3763–3773. doi: 10.1128/mcb.6.11.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Grodzicker T., Stillman B. W. Mutations in the gene encoding the adenovirus early region 1B 19,000-molecular-weight tumor antigen cause the degradation of chromosomal DNA. J Virol. 1984 Nov;52(2):410–419. doi: 10.1128/jvi.52.2.410-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Stillman B. Expression of adenovirus E1B mutant phenotypes is dependent on the host cell and on synthesis of E1A proteins. J Virol. 1987 Feb;61(2):426–435. doi: 10.1128/jvi.61.2.426-435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. T. Programmed cell death: apoptosis and oncogenesis. Cell. 1991 Jun 28;65(7):1097–1098. doi: 10.1016/0092-8674(91)90002-g. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Elwell J. H., Oberley L. W., Goeddel D. V. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989 Sep 8;58(5):923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. 1986 Oct 30-Nov 5Nature. 323(6091):819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Rose K. A., Morris R. G., Steel C. M., Foster E., Spandidos D. A. Rodent fibroblast tumours expressing human myc and ras genes: growth, metastasis and endogenous oncogene expression. Br J Cancer. 1987 Sep;56(3):251–259. doi: 10.1038/bjc.1987.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Venkatesh L., Kuppuswamy M., Chinnadurai G. Adenovirus transforming 19-kD T antigen has an enhancer-dependent trans-activation function and relieves enhancer repression mediated by viral and cellular genes. Genes Dev. 1987 Sep;1(7):645–658. doi: 10.1101/gad.1.7.645. [DOI] [PubMed] [Google Scholar]
- Zerler B., Roberts R. J., Mathews M. B., Moran E. Different functional domains of the adenovirus E1A gene are involved in regulation of host cell cycle products. Mol Cell Biol. 1987 Feb;7(2):821–829. doi: 10.1128/mcb.7.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]







