Abstract
Historically, recruitment of minority subjects for clinical research has been challenging. We developed culturally-tailored recruitment materials for a longitudinal, natural history study of intracerebral hemorrhage (ICH) and trained recruiting coordinators in cultural competence. Of 285 subjects meeting inclusion criteria, 158 (55% of eligible) agreed to participate (60% of eligible blacks vs. 45% of eligible non-blacks, p = 0.02). Of those enrolled, 138 (87%) agreed to participate in the genetic sub-study (86% of blacks vs. 90% of non-blacks enrolled, p = 0.78). Of those subjects who refused enrollment, lack of interest in research (42%) was the most common reason for the study as a whole. A higher rate of enrollment was achieved in blacks vs. non-blacks in this ICH clinical research study employing culturally-tailored recruitment approaches and training of recruitment coordinators to overcome traditional recruitment barriers to research participation in minority patients.
Keywords: Intracerebral hemorrhage, genetics, research recruitment, African Americans
Recruitment of research participants is essential for successful clinical and translational research.(1) Studies that fail to achieve planned enrollments are unable to support planned hypothesis testing, thus compromising scientific relevance and generalizability. A recent evaluation of federally sponsored oncology trials showed that more than 25 percent failed to achieve minimum recruitment goals.(2) Even fewer clinical studies achieve adequate representation of underrepresented minorities.(3–5) Historically, researchers have found that black patients are significantly less likely than whites to participate in clinical research, particularly research involving biomarkers such as neuroimaging or genetic studies.(6–8) These low rates of participation limit the exploration of specific racial and ethnic differences in diseases and delay the development of more effective prevention and treatment strategies. Several factors have been associated with these low rates of minority participation, including that of blacks, in biomedical research. First, potential black subjects may be approached for enrollment less frequently.(9, 10) Second, distrust of research and lack of access to medical care have also been associated with low rates of research participation.(11)
A number of studies have documented the effects of sociological and attitudinal factors such as discrimination, access to care, distrust of the medical community, suspicion of genetic testing, and dislike of the research process. All these factors may affect willingness of minorities, including blacks, to participate in clinical studies.(11–13) Recently, there has been a growing awareness of the importance of minority participation in clinical research.(14) However, few studies have specifically examined and/or reported their recruitment experience, particularly in diseases such as stroke that disproportionately affect minority populations.
Intracerebral hemorrhage (ICH) is a devastating disease with poor prognosis and high mortality rates ranging from 25–50%.(15) ICH is the underlying etiology in 10–15% of all strokes with 75% of those being due to primary (nontraumatic) hemorrhage. Population-based studies have consistently demonstrated that primary ICH is significantly more frequent in underserved populations.(16) The DiffErenCes in the Imaging of Primary Hemorrhage based on Ethnicity or Race (DECIPHER) study is a National Institutes of Health (NIH) funded study designed to investigate whether racial/ethnic differences exist in the underlying risk factors, pathogenesis, and imaging appearance of primary ICH in a predominantly underserved black population. We examined recruitment rates by race, as well as reasons for recruitment or refusal, for subjects enrolled to date in this ICH natural history project and its genetic substudy.
Methods
Study Design
In the DECIPHER study, patients with primary ICH are enrolled and followed for up to 4 years. Inclusion criteria are primary ICH enrolled within 30 days of onset, age ≥ 18, and signed informed consent obtained from the patient or patient’s legally authorized representative. Patients with any of the following are excluded: contraindication to MRI, pregnancy, central nervous system (CNS) tumor or active infection or inflammatory process, CNS arteriovenous malformation, CNS aneurysm, CNS trauma within prior 2 weeks, craniotomy/craniectomy for current ICH, international normalized ratio (INR) > 3.
Patients undergo clinical assessments, magnetic resonance imaging (MRI) and neuropsychological evaluations at enrollment, 30 days, 1 year and 3 years. Brief clinical assessments are performed by phone at 2 years and 4 years. Subjects are also approached for participation in a genetic substudy that includes testing of Aopolipoprotein E (APOE) status. The APOE gene is associated with increased risk of intracerebral hemorrhage. Although subjects of all races and ethnicity are enrolled, we limited the current analysis to black vs. non-black subjects, as there are too few subjects of other races/ethnicities to perform a meaningful analysis or comparison. Moreover, our focus in the current analysis is to explore differences in blacks vs. other races in recruitment rates.
Study Recruitment
All patients admitted with a primary diagnosis of ICH admitted to 5 hospitals in the greater Washington, DC region were screened for eligibility. All recruitment coordinators for the DECIPHER project receive training in cultural competence and all materials are culturally tailored. A number of recruitment strategies have been employed: 1) design of inclusive brochures and written materials at the appropriate literacy level (6th grade), 2) ongoing education of coordinators regarding historical factors that may have contributed to distrust of research as well as disparities in health care, 3) adoption of social marketing strategies addressing social and cultural factors that may affect individual beliefs and behaviors, 4) planned multiple approaches to patients and families, 5) coordinator training in immediately addressing concerns identified by potential subjects. All recruitment materials were reviewed by the community advisory board and materials were modified as appropriate based on their suggestions. Concerns raised by potential participants included cost of participation, loss of privacy, whether family members could provide informed consent and claustrophobia in the MRI scanner.
For all patients eligible for the DECIPHER study, we systematically record reasons for participation or refusal for the overall study as well as reasons for refusal of the genetic sub-study. Data is systematically recorded after each encounter in a structured screening/recruitment log including the number of approaches or interactions; the person approached (patient or proxy), and stated reason for enrollment or refusal. The recruitment log was designed as a one-page checklist to facilitate documentation of recruitment activities. The list of reasons for enrollment or refusal was drawn from documentation created for a previous stroke study conducted in an acute care setting. The recruitment log data was reviewed weekly by the study coordinators and investigators. Table 1 lists the various options for reasons for enrollment or refusal for the overall study and the reasons for refusal for the genetic sub-study.
Table 1.
Recruitment Log Reasons for Participation or Refusal
| Reason for Enrollment In ICH Study |
Reason for Refusal in ICH Study |
Reason For Refusal in Genetics Study |
|---|---|---|
| To benefit others | Patient/LAR not interested in research | Family refusal re poor prognosis |
| To benefit self | Claustrophobia/dislike of MRI/study intervention | Family refusal re uncomfortable deciding for patient |
| Family member wants me to consent | Patient/LAR does not want to make time commitment | Dislike of procedure/needles |
| Other/Unknown; Specify __________ | No direct benefit to patient | Other (please describe): |
| Patient/LAR too tired or overwhelmed | ||
| Privacy concerns/release of PHI | ||
| Patient/LAR refusing due to poor prognosis | ||
| Patient moving out of DC/MD/VA area | ||
| Patient enrolled in competing study |
The main outcome of the analysis is the percentage of eligible participants who have enrolled to date in the main study and the genetic sub-study. Secondary outcomes include the reasons given for participation or refusal.
Analysis
Dichotomous variables were analyzed using Pearson chi-square or Fisher’s exact test as appropriate. Student’s t test was used to analyze mean differences of continuous variables between groups. All analyses were computed using SPSS Version 20(17).
Results
Figure 1 provides a flow chart of screened and enrolled patients. At the time of the current analysis, a total of 892 patients with possible primary ICH had been screened for enrollment in the DECIPHER project. Of these, 417 met inclusion criteria, however, 131 were not consentable (e.g. patient discharged prior to consent, no legally authorized representative reachable). The remaining 286 consentable patients were approached and asked to participate in the study. One hundred and fifty eight agreed to participate (55%), and 128 refused (47%). The demographic characteristics of the study participants and the individuals who refused participation are presented in Table 2. Study participants are significantly younger (p<0.005) and more likely to be black (p<0.04). There were no gender differences (p<0.1). For the overall study, 60% of eligible blacks vs. 47% of eligible non-blacks, (p=0.04) were enrolled (Figure 1).
Figure 1.
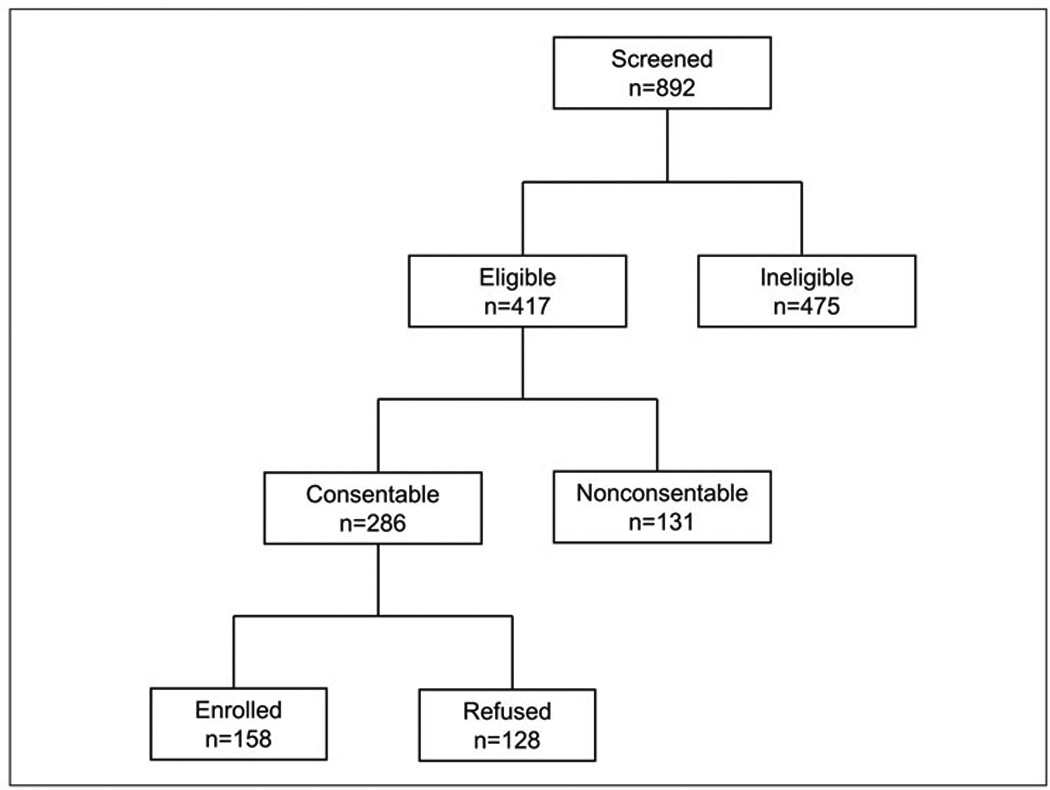
Flow chart of screened and enrolled subjects
Table 2.
Demographic characteristics for enrolled vs. non-enrolled subjects
| Enrolled N=158 |
Refused N=128 |
p value | |
|---|---|---|---|
| Age | 59.2 ± 12.4 | 64.0 ± 15.7 | .005 |
| Black race | 118 (75%) | 80 (62.5%) | .04 |
| Male gender | 89 (56%) | 60 (47%) | .10 |
Of the 158 who consented: 56% were male, 75% were black, mean baseline National Institutes of Health Stroke Scale (NIHSS) was 9 (median 5), and mean baseline MRI ICH volume was 25 cc (median 14 cc). Table 3 provides baseline characteristics for the enrolled cohort overall and by black vs. non-black race.
Table 3.
Demographic Characteristics for Sample and by Black vs. Non-Black Race
| Overall Cohort N=158 |
Black Participants N=119 |
Non-black Participants N=39 |
p value |
|
|---|---|---|---|---|
| Age (yrs) | 59.0 ± 12.54 | 57.5 ± 11.8 | 63.3 ± 13.8 | .02 |
| NIHSS, Mean | 10 ± 10 | 9 ± 10 | 10 ± 8 | .95 |
| Median | 6 | 5 | 7 | |
| Male gender | 88 (56%) | 65 (55%) | 23 (59%) | .65 |
| Education (yrs) | 13.4 ± 2.6 | 13.1 ± 2.5 | 14.2 ± 2.8 | .03 |
| Insurance | ||||
| Private Insurance | 91 (58%) | 68 (57%) | 23 (59%) | .84 |
| Medicare | 42 (27%) | 21 (18%) | 21 (54%) | .0001 |
| Medicaid | 16 (10%) | 16 (10%) | 0 (0%) | .02 |
| No Insurance | 30 (19%) | 24 (20%) | 6 (15%) | .50 |
One hundred thirty-eight (87%) of the 158 enrolled participants also consented to the genetic sub-study: 102 of the 118 blacks (86%) vs. 36 of the 40 non-blacks enrolled (90%), p=0.78) (Figure 1). The mean number of approaches required to obtain consent was 3 (range 1–11). For the patients enrolled, stated reasons for participation were: 1) to benefit others (79%), or 2) to benefit themselves (20%).
Figure 2 provides response frequencies for the reasons for refusal for otherwise eligible and consentable patients. The three most common reasons were 1) lack of interest in research, 2) dislike of MRI, and 3) family refusal due to poor prognosis. The reasons for refusal did not appear to differ by race, however the sample is not large enough to statistically compare differences between the two groups. For the genetic sub-study, the three most common stated reasons for refusal were 1) family refusal due to discomfort with proxy consent process (33%), 2) family refusal due to patient’s poor prognosis (22%), and 3) dislike of procedures/needles.
Figure 2.
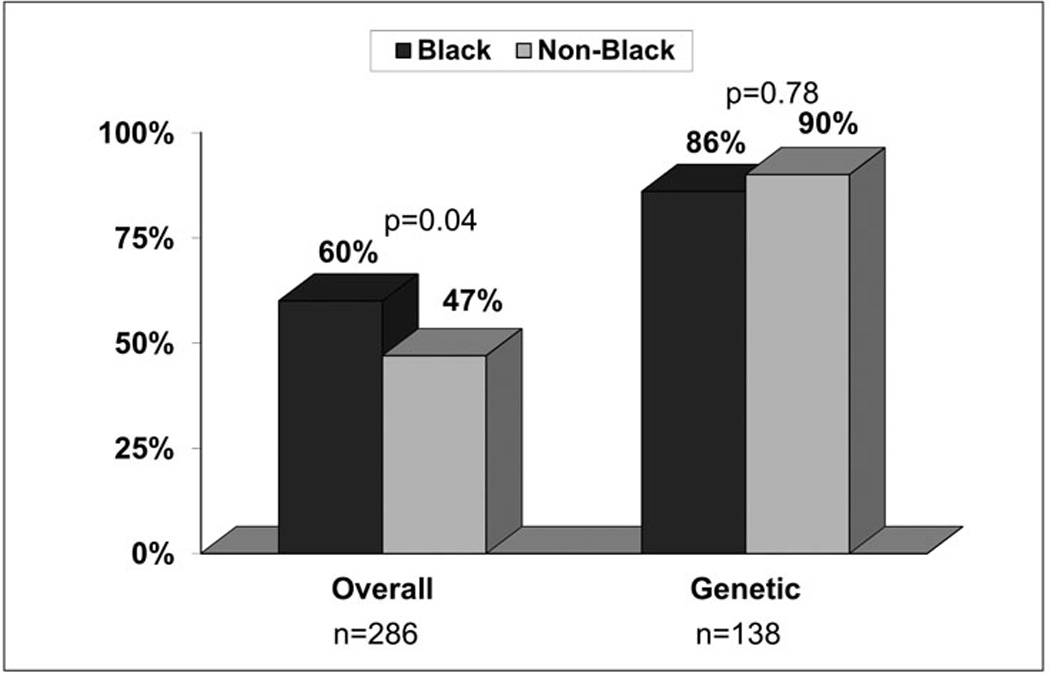
Study recruitment by race
Increased participation in biomedical research is essential to achieving health equity and to decreasing the burden of stroke and other chronic diseases on the black community. Study investigators must not only be familiar with the reasons why black participants are hesitant or unwilling to participate, but also must be able t9o use this information to actively address barriers to participation by tailoring in our study recruitment materials and research procedures. Study materials need to clearly and directly link research participation to the development of new and more effective ways of reducing the impact of stroke in the black community. Study coordinators also must be fully knowledgeable of study procedures and intended outcomes. They should be very comfortable answering questions and addressing concerns as part of the recruitment visit. Finally, study findings should be shared with participants and families as well as the general public so that the benefits of research participation can become more widely known in communities with the greatest risk. Working with local media within the target community to disseminate study findings will help to raise awareness as well as increase interest in future studies.
In conclusion, we achieved greater rates of recruitment of black patients compared to non-blacks in this ICH study. Coordinator training in cultural competence, additional time and resources allocated to the recruitment process to allow for multiple contacts between the recruitment staff and patients and their families, along with trial materials specifically designed to increase trust likely contributed to the overall success of recruitment of black subjects in the DECIPHER project. We also found that both black and white subjects willing to participate in this natural history study were generally willing to participate in genetic research. However, distrust or dislike of research remains an important overall barrier to recruitment.
Figure 3.
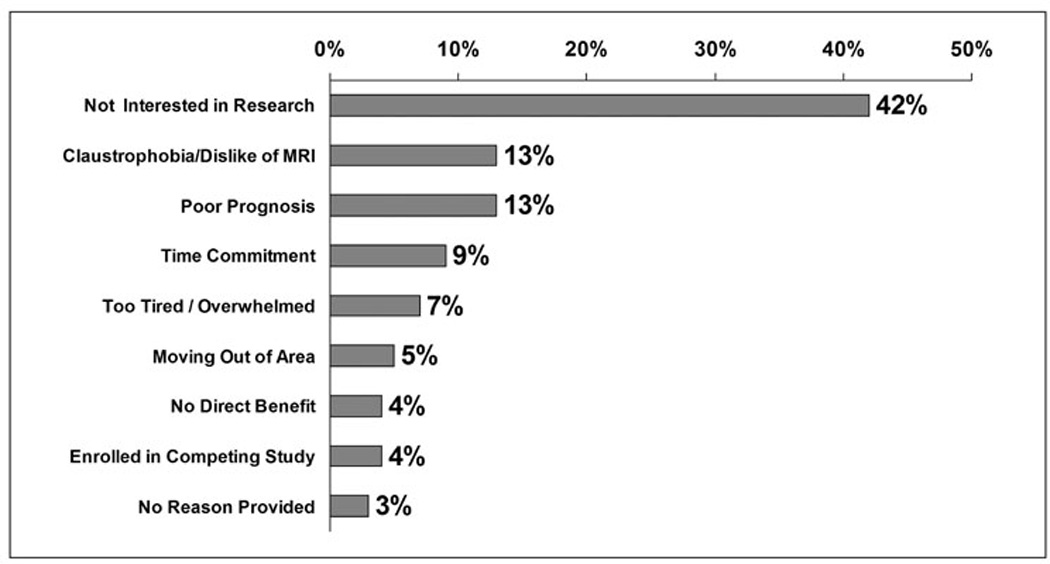
Reasons for Refusal
Acknowledgements
The authors wish to thank the coordinators for study recruitment.
Sources of Funding: This project is supported by Award Number U54NS057405 from the National Institute of Neurological Disorders And Stroke (NINDS) and National Institute on Minority Health and Health Disparities (NIMHD) (U54NS057405). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Footnotes
Disclosures: None
References
- 1.Reis SE, Berglund L, Bernard GR, GA F, Johnson P. Reengineering the national clinical and translational research enterprise:The strategic plan of the National clinical and Translational Sciences Awards Consortium. Academic Medicine. 2010;85:463–469. doi: 10.1097/ACM.0b013e3181ccc877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitterman DR, Cheng SK, Dilts DM, Orwoll E. The prevalence and economic impact of low-enrolling studies at an academic medical center.A. Academic Medicine. 2011;86:1360–1366. doi: 10.1097/ACM.0b013e3182306440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe N. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular trials. Medicine. 2008;87:1–9. doi: 10.1097/MD.0b013e3181625d78. [DOI] [PubMed] [Google Scholar]
- 4.Moye L. Clinical trial minority recruitment: Still an unmet need. J Natl Med Assoc. 2002;94 [PMC free article] [PubMed] [Google Scholar]
- 5.Toerian M, Brookes ST, Metcalfe C, et al. A review of reporting of participant recruitment and retention in RCTs in six major journals. Trials. 2009;10:1–12. doi: 10.1186/1745-6215-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown DR, Topcu M. Willingness to participate in clinical treatment research among older African Americans and whites. Gerontologist. 2003;43:62–72. doi: 10.1093/geront/43.1.62. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson LM, Schwirian PM, Klein EG, et al. Recruitment and retention strategies in longitudinal clinical studies with low-income populations. Contemporary Clinical Trials. 2011;32:353–362. doi: 10.1016/j.cct.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spruill I. Enhancing recruitment of African American families into genetic research: lessons learned from Project SuGar. J Community Genetics. 2010;1:125–132. doi: 10.1007/s12687-010-0017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorelick PBHY, Burnett B, Bonecutter FJ. The recruitment triangle: Reasons why African Americans enroll, refuse to enroll, or voluntarily withdraw from a clinical trial. An interim report from the African-American antiplatelet stroke prevention study (AAASPS) J. Natl Med Assoc. 1998;90 [PMC free article] [PubMed] [Google Scholar]
- 10.Sugarman J, Sitlani C, Andrusiek D, et al. Is the enrollment of racial and ethnic minorities in research in the emergency setting equitable ? Resuscitation. 2009;80:644–649. doi: 10.1016/j.resuscitation.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson VA, Powell-Young YM, Torres ER, Spruill I. A systematic review of strategies that increase recruitment and retention of African American adults in genetic and genomic studies. ABNF. 2011;22:84–88. [PMC free article] [PubMed] [Google Scholar]
- 12.Patterson AR, Davis H, Shelby K, et al. Successful strategies for increasing African American participation in cancer genetic studies: Hopeful signs for equalizing the benefits of genetic medicine. Community Genetics. 2008;11:208–214. doi: 10.1159/000116881. [DOI] [PubMed] [Google Scholar]
- 13.Scharff DP, Mathews KJ, Jackson P, Hoffshuemer J, Martin E, Edwards D. More than Tuskegee:Understanding mistrust about research participation. J health Care for Poor and Underserved. 2010;21:879–897. doi: 10.1353/hpu.0.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu JJ, Davidson E, Sheikh A. Achieving ethnic diversity in trial recruitment. Pharmaceutical Medicine. 2011;25:215–222. [Google Scholar]
- 15.Roger VL, Go AS, Lloyd Jones DM, et al. Heart Disease and Stroke Statistics 2012 Update: A report from the American Heart Association. Circulation. 2011 doi: 10.1161/CIR.0b013e31823ac046. epublished ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruz-Flores S, Rabenstein A, Biller J, et al. Racial-ethnic disparities in stroke care: The American Experience: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2091–2116. doi: 10.1161/STR.0b013e3182213e24. [DOI] [PubMed] [Google Scholar]
- 17.IBM SPSS Version 20 [program] IBM Corporation. 2011. [Google Scholar]
- 18.Froelicher ES, Doolan D, Yerger VB, McGruder CO, Malone R. Combining community participatory research with a randomized clinical trial: The protecting the hood against tobacco (PHAT) smoking cessation study. Heart Lung. 2010;39:50–63. doi: 10.1016/j.hrtlng.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Heiat A, Gross CPKH. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Internal Medicine. 2002;162:1682–1688. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 20.Ejiogu N, Norbeck JH, Mason MA, Cromwell BC, Zonderman AB, Evans M. Recruitment and retention strategies for minority or poor clinical research participants: Lessons from the healthy aging in neighborhoods of diversity across the lifespan study. The Gerontologist. 2011;51:S33–S45. doi: 10.1093/geront/gnr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heiney S, Adams S, Wells L, Johnson H. Evaluation of conceptual framework for recruitment of African American patients with breast cancer. Oncol Nurs Forum. 2010;37:E.160–E.167. doi: 10.1188/10.ONF.E160-E167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joseph G, Dohan D. Recruiting minorities where they receive care: Institutional barriers to cancer clinical trials recruitment in a safety-net hospital. Contemporary Clinical Trials. 2009;30 doi: 10.1016/j.cct.2009.06.009. 55.52-559. [DOI] [PubMed] [Google Scholar]
- 23.Kasner SE, del Giudice A, Rosenberg S, Sheen M, Luciano JM, et al. Who will participate in acute stroke trials? Neurology. 2009;72:1682–1688. doi: 10.1212/WNL.0b013e3181a55fbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phipps E, Harris DBN, Harralson T, Brecher A, Polansky M, Whyte J. Investigation of ethnic differences in willingness to enroll in a rehabilitation research registry: A study of the northeast cognitive rehabilitation research network. American Journal of Physical and Medical Rehabilitation. 2004:875–883. doi: 10.1097/01.phm.0000143436.57173.e1. [DOI] [PubMed] [Google Scholar]


