Figure 1. Ficoll isolation method for hMSCs.
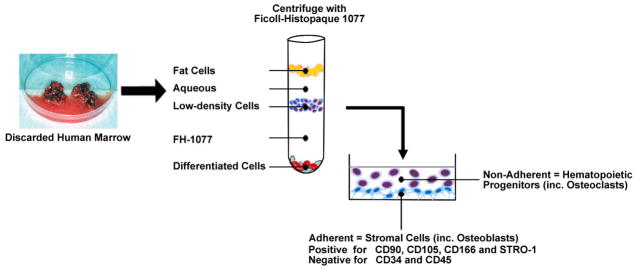
Discarded human bone and marrow tissues are minced in PBS with EDTA to release cells. After passage through a sieve to remove bone particles, cells are collected by centrifugation and resuspension in PBS. Ficoll-Histopaque 1077 (FH-1077, a medium with density of 1.077 g/mL) is carefully added beneath the aqueous cell suspension. Centrifugation separates marrow cells into three major fractions. The fat cells float at the top, and the mature, differentiated erythrocytes and leukocytes sediment at the bottom. Low-density undifferentiated cells accumulate at the interface between the aqueous and FH-1077 layers. This fraction contains progenitors for both hematopoietic and mesenchymal cells. They are collected, washed, and seeded into tissue culture dishes. After 2–3 days incubation, the non-adherent hematopoietic lineage cells, which includes osteoclast progenitors, are removed, allowing the adherent stromal cells to expand. The enriched adherent cells are called hMSCs, which are positive for CD90, CD105, CD166, and STRO-1, and negative for CD34 and CD45.
