Summary
Child maltreatment is a potent stressor associated with neuroendocrine dysregulation and increased risk for mental and physical disorders throughout the lifespan. Gender differences in stress reactivity and adult psychopathology prevalence may be related to sex-specific responsivity to stress. The purpose of this study is to examine whether gender interacts with the stress of maltreatment to produce differential neuroendocrine profiles in children. Participants included 137 maltreated and 110 nonmaltreated low-income, racially and ethnically diverse children (range: 7.9–10.9 years; M= 9.42 years; 52% male) who attended a summer research day camp. Saliva was collected 3 times across the day for 5 days for cortisol and dehydroepiandosterone (DHEA) analysis. Department of Human Services records were examined to determine the type, severity, chronicity, onset, and recency of maltreatment for children in the maltreated group. Significant interactions between gender and maltreatment pervasiveness predicted diurnal cortisol, DHEA, and cortisol/DHEA ratio levels. Elevated daily cortisol levels were reported for boys compared to girls in the group with more pervasive maltreatment. Boys with less pervasive maltreatment had lower DHEA and higher cortisol/DHEA ratio levels than girls with similar experiences, nonmaltreated boys, and boys with more pervasive maltreatment. Further results are consistent with down-regulation of cortisol production in girls with more pervasive maltreatment and girls who experienced maltreatment that was early onset and not recent. The effectiveness of interventions for maltreated children may be improved with greater knowledge of how maltreatment differentially affects neuroendocrine regulation by gender.
Keywords: Child Maltreatment, Cortisol, DHEA, Sex Differences, Health Disparities
Introduction
Chronic stress in childhood contributes to progressive wear and tear on the body that can have lasting effects on mental and physical health (Shonkoff, Boyce, & McEwen, 2009). Child maltreatment significantly increases the risk for psychopathology across the lifespan (Cicchetti & Valentino, 2006), and atypical hypothalamic-pituitary-adrenal (HPA) regulation following maltreatment in childhood may contribute to this heightened vulnerability to poor mental health (van Goozen & Fairchild, 2008). Increasing evidence indicates potential gender differences in neuroendocrine profiles in response to traumatic stress, especially in adults. Less work has been done in children, but different groups have hypothesized about stress system regulation in response to serious childhood stressors, such as maltreatment. Interestingly, both hyper and hypoactivation of the stress system have been documented in response to traumatic stress, and understanding patterns of neuroendocrine activity may elucidate mechanisms of stress system regulation. In this study, we will test the opposing hypotheses of the Adaptive Calibration Model (ACM) (Del Giudice, Ellis, & Shirtcliff, 2011) and Kajantie and Phillips (2006), which predict differential responses to traumatic stress based on gender.
The ACM (Del Giudice et al., 2011) proposes that evolution may have led to stress system recalibration around the time of puberty due to differential life history strategies between males and females. Increasing sexual differentiation and rising adrenal hormones in middle childhood are hypothesized to underlie sex differences in stress reactivity and regulation, with environmental stress predicting the amount of divergence between sexes. Under a moderate amount of stress, individuals are hypothesized to have better physiological regulation and optimal engagement with the environment, resulting in only moderate sex differences. In highly stressful or threatening situations, boys are predicted to become more callous and unemotional, traits linked to low stress responsivity. Girls are predicted to become fearful and anxious and to develop internalizing symptoms, which are associated with high sympathetic and HPA reactivity. However, studies testing the ACM’s gender hypothesis have not yet been conducted. In this study, we will test the ACM’s hypothesis that traumatic stress will lead to low stress system responsivity in males compared to females.
Kajantie and Phillips (2006) would predict blunted cortisol levels for women who have experienced early trauma. A review of the literature suggests lower HPA and autonomic responses to stress in adult women versus men, with significant variation depending on the menstrual phase in women (Kajantie & Phillips, 2006). They suggest that modifiers such as estrogen, arginine vasopressin, and corticosteroid-binding globulin could be responsible for the sex differences observed in adults. The reason for physiological hyporesponsiveness in women could be an evolutionary pressure to protect a potential fetus from excess glucocorticoids, especially due to research reporting attenuated HPA responses in pregnant women.
Gender Differences in Neuroendocrine Profiles
There is evidence that differences in HPA reactivity and basal activity, which result in production of the hormones cortisol and dehydroepiandosterone (DHEA) in the adrenal glands, are apparent between males and females, especially after puberty. In pre-pubertal children, there are generally no differences in baseline cortisol levels, but as children approach puberty, girls tend to mature faster and may show higher cortisol levels than boys (Netherton et al., 2004; Jessop & Turner-Cobb, 2008). Post-puberty, men are more likely to have elevated cortisol responses to stress than women (for review, Kudielka & Kirschbaum, 2005). On the other hand, DHEA levels are positively correlated with age, but not gender (Netherton et al., 2004).
Effects of Child Maltreatment on Cortisol and DHEA
Child maltreatment is a potent moderator of stress-mediating systems. However, the literature on the exact effects of maltreatment on the HPA axis is mixed, and discrepancies are likely due to differential maltreatment experiences, environmental risk and protective factors, and concurrent psychiatric disorders (for review, see Tarullo & Gunnar, 2006; Van Voorhees & Scarpa, 2004). Depressed and non-depressed men and women maltreated as children have shown increased cortisol and adrenocorticotropic hormone (ACTH) reactivity to challenge (Heim et al., 2002; Heim et al., 2008). However, some studies in adults maltreated as children report attenuated cortisol and ACTH responses to stress in adults without psychopathology (Carpenter et al., 2007) and women with PTSD (Bremner et al., 2007).
The pattern of HPA axis regulation following maltreatment is often unclear in studies of children as well. For example, physical and sexual abuse have been associated with high morning cortisol levels in children, and those who have suffered multiple types of abuse are more likely to exhibit high morning and afternoon cortisol levels (Cicchetti & Rogosch, 2001a). Children with PTSD secondary to trauma have also exhibited elevated cortisol levels compared to controls (De Bellis et al., 1999). However, some physically abused children show low cortisol and a flattened diurnal slope compared to nonmaltreated children (Cicchetti & Rogosch, 2001a), and adolescent females with PTSD secondary to rape have demonstrated low cortisol levels (Bicanic et al., 2012). Several studies have reported attenuated cortisol responses to stressors in maltreated boys and girls (Ouellet-Morin et al., 2011) and maltreated females specifically (De Bellis et al., 1994; MacMillan et al., 2009). Internalizing and externalizing symptomology may also play a role in HPA axis regulation. For example, school-age children experiencing physical and/or sexual abuse before age 5 have demonstrated an attenuated diurnal cortisol slope when accompanied by concurrent internalizing symptoms (Cicchetti, Rogosch, Gunnar, & Toth, 2010). In addition, maltreated children may show high or low levels or flattening of the diurnal slope depending on the presence of internalizing or externalizing symptoms (Cicchetti & Rogosch, 2001b). In maltreated individuals, low cortisol could be related to subsequent PTSD, while high cortisol could be concurrent with depressive disorders (McCrory et al., 2011).
A review by Tarullo and Gunnar (2006) noted increased basal cortisol levels in maltreated children with internalizing problems while adults who were maltreated as children tend to show hyposecretion of cortisol and increased ACTH responsiveness, with puberty targeted as a potential time of stress system reorganization in these individuals. A recent study demonstrated that sexually abused girls who exhibited high cortisol levels in childhood showed attenuation in adolescence and transitioned to low cortisol levels in adulthood (Trickett, Noll, Susman, Shenk, & Putnam, 2010), indicating a switch to hypocortisolism over time that could be due to receptor down-regulation following a sustained ACTH drive (Fries, Hesse, Hellhammer, & Hellhammer, 2005). Timing of maltreatment likely impacts the temporal regulation of the stress system, and studies have not yet examined gender differences in stress system regulation based on the onset and recency of maltreatment.
DHEA is an adrenal steroid that has anti-glucocorticoid properties and may protect the body from high levels of cortisol (Charney, 2004). DHEA hyposecretion is related to major depression in adolescents and may be a risk factor for psychopathology (Goodyer, Park, Netherton, & Herbert, 2001). Although maltreatment status does not independently predict DHEA levels (Bremner et al., 2007; Cicchetti & Rogosch, 2007), there is evidence that morning and afternoon DHEA levels are related to resilient functioning, with highly resilient maltreated children showing an unexpected rise in DHEA across the day (Cicchetti & Rogosch, 2007).
A high cortisol/DHEA ratio has been likened to a chronic stress response. Higher cortisol/DHEA levels have been noted in depressed adults, with the ratio positively related to the length of the current depressive episode (Young, Gallagher, & Porter, 2002). In 8- to 16-year-olds, high ratio levels in the evening predicted the maintenance of major depression (Goodyer, Herbert, & Altham, 1998). However, a high cortisol/DHEA ratio may not be deleterious in all circumstances. One study reported that higher cortisol/DHEA levels were associated with more resilient functioning in both maltreated and non-maltreated children (Cicchetti & Rogosch, 2007). As a result, a high cortisol/DHEA ratio may not necessarily be maladaptive, and further research is needed to clarify its relationship to current functioning.
In the current investigation, child maltreatment is hypothesized to interact with gender to produce differences in diurnal cortisol, DHEA, and cortisol/DHEA ratio levels. A significant body of research reports hyposecretion of cortisol in maltreated females (De Bellis et al., 1994; MacMillan et al., 2009) and hyperreactivity to psychosocial stress in adult males (Heim et al., 2008; Kajantie & Phillips, 2006), in line with the Kajantie and Phillips (2006) hypotheses. On the other hand, no studies to date have tested the gender hypotheses for HPA axis regulation proposed by the ACM. As a result, it is predicted that girls with more pervasive maltreatment will have lower diurnal cortisol, DHEA, and cortisol/DHEA levels than nonmaltreated girls. Conversely, boys are hypothesized to show increased cortisol and cortisol/DHEA levels and decreased DHEA compared to maltreated girls and nonmaltreated boys. In addition, neuroendocrine levels will be examined based on the timing of maltreatment to inform understanding of temporal processes in stress system regulation that may differ by gender. It is predicted that with earlier onset and more recent maltreatment, girls will display a pattern of neuroendocrine hyporeactivity to nonmaltreated girls and boys at similar levels of maltreatment, while boys will show hyperreactivity compared to nonmaltreated boys and girls at similar levels of maltreatment. These hypotheses are in line with Kajantie and Phillips (2006) and in contrast to the ACM model predictions. Both perspectives will be tested to develop a more complete understanding of gender differences in response to stress.
Methods
Participants
Participants included 247 children (118 females, 129 males) attending a research summer camp program for low-income maltreated (n = 137) and nonmaltreated children (n = 110). The average age of the children was 9.42 years old (SD = 0.88, range = 7.9–10.9 years). The sample was diverse both racially (62.3% Black, 21.1% White, 16.6% biracial or other race) and ethnically (24.3% Latino). Research staff obtained informed consent from parents of all children for their child’s participation in the summer camp and for permission to examine any Department of Human Services (DHS) records of the family. The Institutional Review Board of the University of Rochester approved these methods and procedures.
A DHS liaison recruited children in the maltreatment group by examining Child Protective Services reports to identify children who had experienced maltreatment. Children who were in foster care were not recruited for this study. Eligible families were contacted by the DHS liaison who explained the study and obtained signed permission for their contact information to be shared with research staff if they were interested in participating. The sample was representative of families receiving DHS services. After recruitment, trained research staff and a clinical psychologist conducted reviews of all DHS records for each family and coded maltreatment information using the Barnett, Manly, and Cicchetti (1993) child maltreatment classification system. Mothers were also interviewed with the Maternal Child Maltreatment Interview (Cicchetti, Toth, & Manly, 2003). Staff were instructed to code using all available information and not to rely on DHS determinations.
Because the majority of maltreating families reside in the lower-SES (National Incidence Study; Sedlak et al., 2010), nonmaltreating families receiving Temporary Assistance to Needy Families were recruited in order to make comparisons with SES held constant. Eligible nonmaltreating families were contacted by the DHS liaison who described the study and obtained signed permission for their contact information to be released to research staff for recruitment. All mothers were informed that they could refuse study participation without consequence to their DHS services or any other penalty. Families were asked to participate after staff confirmed through comprehensive DHS record searches that there was no documented child maltreatment. Families were excluded from the nonmaltreatment group if they received preventative DHS services due to concerns about maltreatment. Qualified research assistants interviewed the mothers of children recruited for the nonmaltreatment group using the Maternal Child Maltreatment Interview (Cicchetti, Toth, & Manly, 2003) to inquire about any maltreatment the child may have experienced that was not reported to DHS. To assure that all information had been evaluated, research assistants also examined DHS records in the year after camp participation.
Families of children in the maltreated and nonmaltreated groups did not differ on a number of demographic characteristics (Table 1). These include maternal education, χ2 (1, N = 244) = 0.36, p > .05, marital status, χ2 (3, N = 244) = 2.32, p > .05, total family income including public assistance t (239) = 1.17, p > .05, and family history of receiving public assistance, χ2 (1, N = 244) = 0.78, p > .05. In this sample, the race of participants did not differ by maltreatment status, χ2 (2, N = 236) = 3.11, p > .05, or sex, χ2 (2, N = 236) = 1.79, p > .05. As a result, racial dissimilarities between groups cannot be the cause of any neuroendocrine differences in the sample.
Table 1.
Family Demographic Characteristics
| Maltreated
|
Nonmaltreated
|
|||||
|---|---|---|---|---|---|---|
| M | SD | % | M | SD | % | |
| Marital Status | ||||||
| Never married | 34.3 | 37.4 | ||||
| Married | 17.5 | 21.5 | ||||
| Living with partner | 25.5 | 17.8 | ||||
| No longer married | 22.6 | 23.4 | ||||
| Maternal education | ||||||
| Did not graduate high school | 45.3 | 41.4 | ||||
| Total family income | ||||||
| $1000s including public assistance | 25.88 | 14.90 | 28.15 | 14.94 | ||
| Family history of receiving public assistance | 99.3 | 100 | ||||
Note: All group contrasts were not significant.
Procedures
Day Camp Procedure
Children were randomly assigned to peer groups of 10, all of whom were the same sex and age. In each of these groups, 5 children were maltreated and 5 were nonmaltreated. Three trained camp counselors led each group, and they were not aware of maltreatment status or the study hypotheses. During the week, children participated in recreational activities. Once child assent was obtained, children participated in assessments conducted by skilled research assistants, who also collected child saliva samples for cortisol and DHEA assay (for detailed descriptions of camp procedures, see Cicchetti & Manly, 1990). Research assistants conducted interviews with the caregiver of each child to obtain family demographic information and assess child psychiatric history and health status. At the end of each week of camp, counselors assessed behavioral symptomatology by completing the Teacher Report Form (TRF; Achenbach, 1991). Counselors evaluated children in their group at the end of the week for each cohort of children. At the time of assessment, counselors had spent an average of 35hr of observation and interaction with the children. The TRF was used because counselors could observe behaviors that occur in classroom settings in the camp context. Research assistants at all stages were unaware of maltreatment status and study hypotheses.
Measures
Maltreatment Classification System (MCS)
The MCS (Barnett et al., 1993) is used to assess each child’s experiences of maltreatment and allows researchers to make independent evaluations of maltreatment using DHS records. The MCS classifies the following maltreatment parameters: subtypes that each child experienced, frequency of occurrence, subtype severity, and developmental periods of occurrence. This information is used to define maltreatment recency, onset, and chronicity. Maltreatment subtypes include neglect, emotional maltreatment, physical abuse, and sexual abuse. The MCS provides operationally defined inclusion and exclusion criteria to allow researchers to code for each subtype. Neglect is defined as the failure to provide for the child’s basic physical necessities, including sufficient food, clothing, shelter, and medical treatment. Lack of supervision, moral–legal neglect, and educational neglect are also classified under neglect in the MCS. Emotional maltreatment comprises extreme avoidance of providing for children’s basic emotional needs, including psychological safety and security. Belittling and ridiculing the child, extreme negativity and hostility, child abandonment, suicidal or homicidal threats, and extreme negativity and hostility are examples of emotional maltreatment. Physical abuse involves nonaccidental physical injury such as bruises, welts, burns, choking, and broken bones. Sexual abuse includes attempted or actual sexual contact between the child and caregiver for the caregiver’s sexual gratification or financial gain. Sexual abuse can range from exposure to pornography or adult sexual behavior to sexual contact and fondling to forced intercourse with the child.
The total number of subtypes of maltreatment each child experienced was added to create a sum of subtype variable with a possible range from 0–4. A 5-point scale from the MCS is used to rate the severity of each of the 4 possible subtypes of maltreatment experienced. The severity ratings for each subtype were added, yielding a sum of maltreatment severity score ranging from 0–20. The developmental periods during which maltreatment occurred were recorded. These include infancy, toddlerhood, preschool age, early school age, and late school age. The total number of developmental periods in which maltreatment occurred was summed to create a score for each child that ranged from 0–5. Children whose maltreatment onset was in infancy, toddlerhood, or preschool were defined as early onset, and those whose onset was early or late school age were defined as late onset. Children whose most recent experience of maltreatment was in infancy, toddlerhood, or preschool were defined as not recent, and those whose latest experience was early or late school age were defined as recent. For analysis, children were divided into three groups based on developmental periods of maltreatment: early onset/not recent, early onset/recent, and late onset/recent.
For the sum of subtypes, children were divided into 3 groups: nonmaltreated = 0, 1 subtype = 1, 2+ total subtypes = 2. For the sum of severity, children were divided into 3 groups: nonmaltreated = 0, less severe maltreatment (sum of severity score between 1–6) = 1, and more severe maltreatment (severity sum of 7–12) = 2. For the sum of developmental periods of maltreatment, children were divided into 3 groups: nonmaltreated = 0, 1 developmental period of maltreatment = 1, or 2+ periods of maltreatment = 2. These variables were significantly correlated. Within the maltreated group, severity and sum of subtypes were correlated at r = .56, severity and duration at r = .45, and sum of subtypes and duration at r = .58. In order to create a composite maltreatment variable characterizing variation in maltreatment pervasiveness for the analyses, the three variables (sum of subtypes, sum of severity, and sum of developmental periods of maltreatment), which were each scored from 0–2, were added to create a variable ranging from 0–6. There were 109 nonmaltreated children (score of 0 on the pervasiveness variable), 52 children with less pervasive maltreatment (score of 1–3), and 73 children with more pervasive maltreatment.
The MCS has demonstrated reliability and validity in maltreatment classification in numerous studies (see, e.g., Bolger, Patterson, & Kupersmidt, 1998; Manly, 2005). Skilled research staff and a clinical psychologist coded DHS records using the MCS, and all coders achieved adequate reliability before coding for this investigation. Kappas ranged from 0.90 to 1.00 for the presence of each of the maltreatment subtypes, and intraclass correlations ranged from 0.83 to 1.0 for severity ratings of individual subtypes of maltreatment. In this investigation, 76.6% of maltreated children had experienced neglect, 48.9% emotional maltreatment, 23.4% physical abuse, and 8.8% experienced sexual abuse. Neglect and emotional maltreatment were highly prevalent in the sample, while physical and sexual abuse happened less often. In this study, 51.9% of children who were subjected to maltreatment experienced two or more subtypes (M = 1.67, SD = 0.73), which is consistent with other samples (Manly et al., 2001). In the maltreated group, 46.4% had experienced early onset/not recent maltreatment, 27.2% were in the early onset/recent maltreatment group, and 26.4% were in the late onset/recent maltreatment group (see Table 2).
Table 2.
Maltreatment variables
| Variable | M | SD | Range | % |
|---|---|---|---|---|
| Less Pervasive Maltreatment | ||||
| Sum of Subtypes | 1 | 0 | 1 | |
| Sum of Severity | 2.73 | 1.17 | 1–5 | |
| Developmental Periods | 1 | 0 | 1 | |
| More Pervasive Maltreatment | ||||
| Sum of Subtypes | 2.16 | 0.60 | 1–3 | |
| Sum of Severity | 6.25 | 2.40 | 2–12 | |
| Developmental Periods | 1.90 | 0.67 | 1–4 | |
| Early Onset/Not Recent Maltreatment | 46.4 | |||
| Early Onset/Recent Maltreatment | 27.2 | |||
| Late Onset/Recent Maltreatment | 26.4 | |||
Note: Calculations based on maltreated group only. Developmental periods refers to the total number of developmental periods of maltreatment a child experienced (e.g., chronicity). Onset/recency based on the entire maltreated group.
Teacher Report Form (TRF)
The TRF is a widely used assessment consisting of 118 items rated for frequency of various symptoms and behavioral disturbances. Items load onto eight symptom scales and three summary scales. Symptom scales include withdrawn, somatic complaints, anxiety/depression, social problems, thought problems, attention problems, delinquent behavior, and aggressive behavior. Summary scales include internalizing behavior, externalizing behavior, and total behavior problems. Among pairs of raters, average intraclass correlations were 0.68 for internalizing, 0.87 for externalizing, and 0.88 for total behavior problem scores.
Cortisol and DHEA
Children provided saliva samples at the same time for each of the five days of the camp week. Samples were collected when the child arrived at camp at 0900h, before lunch at 1200h, and before they departed at 1600h. To account for the high variability in cortisol levels during the first hour post-wakening, all saliva samples were collected after the 45-min bus ride to camp and initial staff greeting. Saliva was assayed for cortisol for each day and time during the week that it was collected. Due to less variability in DHEA levels, DHEA was assayed from the three daily saliva samples only on Tuesday and Thursday. Research assistants obtained these samples using a standardized procedure (Granger, Schwartz, Booth, Curran, & Zakaria, 1999). Children did not consume food or drink within 30 min of sample collection. Children chewed Trident® sugarless original flavor gum to stimulate saliva flow and passively drooled through a short straw into a 20-ml plastic vial. Samples were immediately frozen and stored at −80°C. Samples for the week were shipped overnight on dry ice for next day delivery to Salimetrics Laboratories (State College, PA). Each sample was thawed and then processed by placing four to five 1-ml aliquots into 1.8-ml cryogenic storage vials which were frozen at −80°C. Samples were thawed to room temperature upon assay and centrifuged at 3000 rpm for 15 min. The clear top phase was pipetted into appropriate test tubes/wells.
Salivary cortisol (μg/dl) was assayed using an enzyme immunoassay kit (Salimetrics). This kit is commercially available and uses 25 μl of saliva. Its lower limit of sensitivity is 0.007 μg/dl (range up to 1.8 μg/dl) with average intra- and interassay coefficient of variation of <5.0% and 10.0%, respectively. Salivary DHEA (pg/ml) was processed using an enzyme immunoassay kit (Salimetrics) that uses 50 μl of saliva. Its lower limit of sensitivity is 10.0 pg/ml (range up to 1000 pg/ml) with average intra- and interassay coefficient of variation equaling <5.0% and 15.0%, respectively. The antibody used had no measurable crossreactivity with DHEA. To correct for extreme outliers, values greater than +2 SD were winsorized to the +2 SD level. There were 32/1042 morning cortisol samples, 35/1115 afternoon cortisol samples, 22/409 morning DHEA samples, and 23/426 afternoon DHEA samples that were greater than 2 SD from the mean. Morning, noon, and afternoon cortisol values for each day were averaged to obtain mean morning, noon, and afternoon cortisol values, respectively. Morning, noon, and afternoon DHEA values for the two days were averaged to obtain mean morning, noon, and afternoon DHEA values, respectively. All values were log transformed prior to analyses to account for the significant skew in cortisol and DHEA values for each day and time of sample.
The morning cortisol/DHEA ratio was calculated as the mean of the winsorized morning cortisol values (converted to mol/mL) divided by the mean of the winsorized morning DHEA values (mol/mL). The same procedure was used to compute the noon and afternoon cortisol/DHEA ratios. The ratios for morning, noon, and afternoon were then log-transformed for analysis.
Results
Data Analytic Plan
To predict neuroendocrine outcomes, interactions between gender and maltreatment groups (nonmaltreated, less pervasive maltreatment, more pervasive maltreatment) were analyzed by two-way repeated measures analysis of covariance (ANCOVA). Maltreatment groups were created using a composite variable that included the sum of subtypes of maltreatment, sum of the severity of each maltreatment subtype experienced, and the number of developmental periods in which maltreatment occurred. To determine whether the periods of onset and recency of maltreatment affect hormone outcomes, two-way repeated measures ANCOVA were conducted for each hormone outcome using maltreatment onset/recency groups (early onset/not recent, early onset/recent, and late onset/recent) and gender. Pairwise comparisons with Bonferroni adjustments were run to determine which groups differed from one another. In order to reduce Type I error, analyses were first run for cortisol/DHEA levels, and if significant, further analyses were conducted to see if differences were due to cortisol, DHEA, or both hormones. As there were no a priori hypotheses about interactions for particular times of the day or the slope of hormone levels across the day, diurnal hormone output is the focus of the results and graphs.
Due to research reporting age as a significant predictor of morning cortisol in girls and morning and evening DHEA levels in both girls and boys, age was included as a covariate in all analyses (Netherton et al., 2004). In addition, research indicates that maltreatment affects hormone outcomes in relation to the presence of internalizing and externalizing symptoms (Cicchetti & Rogosch, 2001b). To ensure that associations between maltreatment, gender, and hormone outcomes were not due to internalizing and externalizing symptomology, all repeated measures ANCOVA analyses were run including internalizing and externalizing symptoms as covariates.
Due to missing data from children who did not attend enough days of camp, 13 children were excluded from analyses, leaving 234 children in the sample. Descriptive statistics for the less pervasive and more pervasive maltreatment groups are shown in Table 2.
Cortisol/DHEA ratio
Maltreatment Pervasiveness
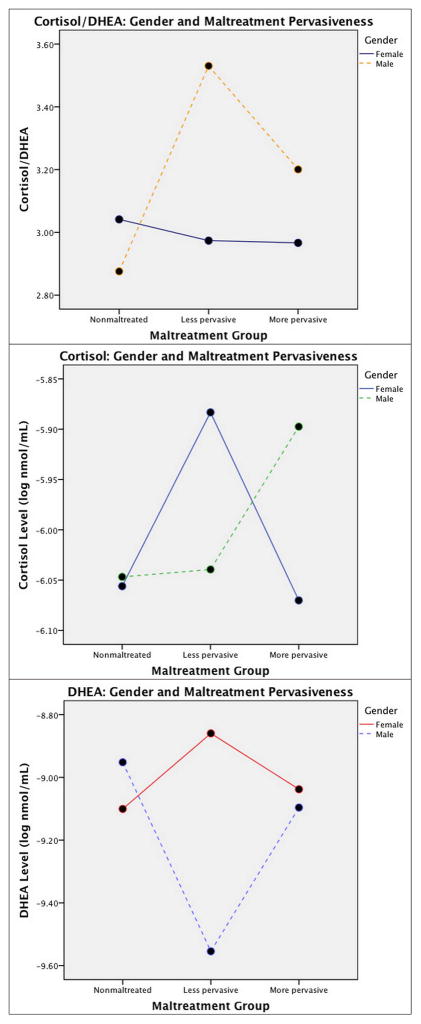
The results of a 3 (nonmaltreated vs. less pervasive maltreatment vs. more pervasive maltreatment) x 2 (male vs. female) repeated measure ANCOVA of morning, noon, and afternoon cortisol/DHEA levels controlling for age, internalizing behaviors, and externalizing behaviors yielded a significant maltreatment pervasiveness x gender interaction for cortisol/DHEA averaged across the day, F(2, 216) = 4.77, p < .01 (Figure 1a). In the group with less pervasive maltreatment, boys have significantly higher cortisol/DHEA levels than girls, F(1, 45)= 8.26, p < .01, but there were no gender differences in the nonmaltreated and more pervasive maltreatment groups, F(1, 99)= 2.14, p > .05, and F(1, 66)= 2.21, p > .05. There was a significant group difference in cortisol/DHEA for boys, F(2, 105)= 6.91, p < .01, with significantly higher cortisol/DHEA levels for boys who experienced less pervasive maltreatment than for nonmaltreated boys. However, there were no maltreatment group differences in cortisol/DHEA for girls, F(2, 108) = .20, p > .05.
Figure 1.
Average daily a) cortisol/DHEA, b) cortisol, and c) DHEA levels for nonmaltreated, less pervasively maltreated, and more pervasively maltreated children by gender. Cortisol/DHEA is expressed as a ratio, and cortisol and DHEA values are represented in log-transformed nmol/mL.
Maltreatment Onset and Recency
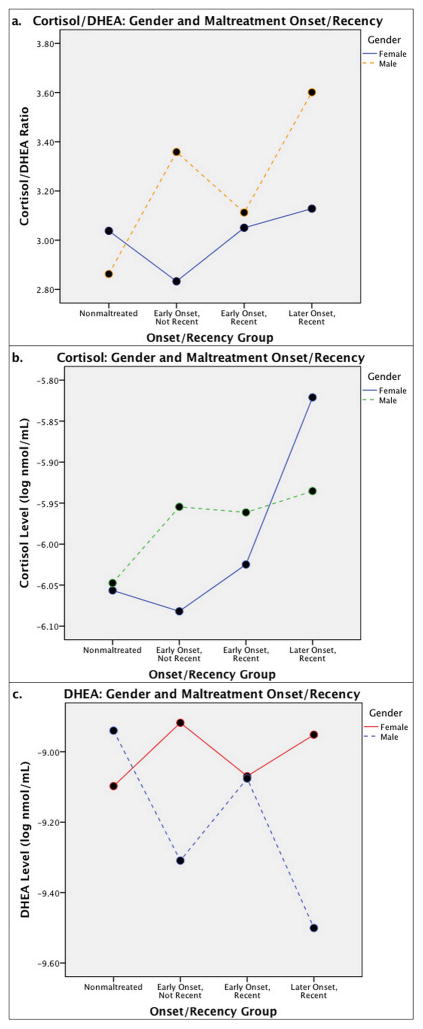
A 4 (nonmaltreated, early onset/not recent maltreatment, early onset/recent maltreatment, late onset/recent maltreatment) x 2 (male vs. female) repeated measures ANCOVA of morning, noon, and afternoon cortisol/DHEA levels controlling for age, internalizing behaviors, and externalizing behaviors produced a significant maltreatment onset/recency x gender interaction for cortisol/DHEA averaged across the day, F(3, 214) = 3.64, p < .05 (Figure 2a). For boys, post-hoc comparisons with Bonferroni adjustments revealed that the early onset/not recent maltreatment and late onset/recent groups had significantly higher daily cortisol/DHEA ratios than the nonmaltreated group, F(3, 104) = 4.98, p < .01. There were no differences between onset/recency groups for girls, F(3, 107) = .76, p > .05. Within the early onset/not recent maltreatment group, boys had higher cortisol/DHEA ratios than girls, F(1, 52) = 8.50, p < .01, but there were no gender differences in the early onset/recent maltreatment group, F(1, 27) = .07, p > .05. In the late onset/recent maltreatment group, boys had marginally higher cortisol/DHEA than girls, F(1, 27) = 3.55, p = .07.
Figure 2.
Average daily a) cortisol/DHEA, b) cortisol, and c) DHEA levels for nonmaltreated, early onset/not recent maltreatment, early onset/recent maltreatment, and late onset/recent maltreatment groups by gender. Cortisol/DHEA is expressed as a ratio, and cortisol and DHEA values are represented in log-transformed nmol/mL.
Cortisol
Maltreatment Pervasiveness
A 3 (nonmaltreated vs. less pervasive maltreatment vs. more pervasive maltreatment) x 2 (male vs. female) repeated measures ANCOVA of morning, noon, and afternoon cortisol levels that controlled for age, internalizing behaviors, and externalizing behaviors produced an interaction between time of day and gender, F(1, 220) = 8.25, p < .01, with girls having a greater decline in cortisol across the day compared to boys. A significant maltreatment pervasiveness x gender interaction was observed in cortisol averaged across the day, F(2, 220) = 3.17, p < .05 (Figure 1b). Pairwise comparisons revealed that girls with less pervasive maltreatment had higher levels of cortisol than nonmaltreated girls, p < .05, and girls with more pervasive maltreatment experiences, p < .05. There was not a significant difference in cortisol levels between the nonmaltreated and more pervasive maltreatment groups, p > .05. There were no differences between maltreatment groups for boys, p > .05. Within the more pervasive maltreatment group, boys had higher cortisol levels than girls, F(1, 67) = 4.96, p < .05. There were no gender differences in cortisol levels in the nonmaltreated group and the group with less pervasive maltreatment, F(1, 101) = .20, p > .05, and F(1, 46) = 1.96, p > .05, respectively.
Maltreatment Onset and Recency
A 4 (nonmaltreated, early onset/not recent maltreatment, early onset/recent maltreatment, late onset/recent maltreatment) x 2 (male vs. female) repeated measures ANCOVA of morning, noon, and afternoon cortisol levels controlling for age, internalizing behaviors, and externalizing behaviors was not significant, F(3, 218) = .84, p > .05 (Figure 2b). However, post-hoc analyses with Bonferroni adjustments indicated that for girls, the late onset/recent maltreatment group had significantly higher cortisol levels than the nonmaltreated and early onset/not recent groups, F(3,108) = 3.67, p < .05. There were no gender differences within the nonmaltreated, early onset/not recent, early onset/recent, and late onset/recent groups, p > .05. For boys, there were no differences in cortisol levels between maltreatment onset/recency groups, F(3, 107) = , p > .05.
DHEA
Maltreatment Pervasiveness
The results of a 3 (nonmaltreated vs. less pervasive maltreatment vs. more pervasive maltreatment) x 2 (male vs. female) repeated measures ANCOVA of morning, noon, and afternoon DHEA levels controlling for age, internalizing behaviors, and externalizing behaviors yielded an interaction between maltreatment pervasiveness and gender for DHEA averaged across the day, F(2, 217) = 7.23, p = .001 (Figure 1c). Within the group with less pervasive maltreatment, boys had lower DHEA than girls, F(1, 45) = 12.14, p = .001, but no gender differences were found in the nonmaltreated group and the group with more pervasive maltreatment, F(1, 100) = 1.44, p > .05, and F(1, 66) = .133, p > .05, respectively. For boys, the group with less pervasive maltreatment had significantly lower DHEA than the group with more pervasive maltreatment and the nonmaltreated group, p < .05, but girls showed no differences between maltreatment groups, p > .05.
Maltreatment Onset and Recency
A 4 (nonmaltreated, early onset/not recent maltreatment, early onset/recent maltreatment, late onset/recent maltreatment) x 2 (male vs. female) repeated measures ANCOVA of morning, noon, and afternoon cortisol/DHEA levels controlling for age, internalizing behaviors, and externalizing behaviors produced a significant maltreatment onset/recency x gender interaction for DHEA averaged across the day, F(3, 215) = 3.40, p < .05 (Figure 2c). For boys, post-hoc analyses with Bonferroni adjustments indicated a significant difference between the nonmaltreated and late onset/recent maltreatment groups with lower DHEA levels for the late onset/recent group, F(3, 105) = 3.89, p < .05. Boys in the early onset/not recent group had marginally lower DHEA levels than boys in the nonmaltreated group, p = .063. All other group contrasts for boys were nonsignificant, p > .05. There were no group differences between onset/recency groups for girls, F(3, 107) = .68, p > .05. In the early onset/not recent group, girls have significantly higher DHEA than boys, F(1, 52) = 5.24, p < .05. In the late onset/recent maltreatment group, girls have marginally higher DHEA levels than boys, F(1, 27) = 3.69 p = .065. There were no gender differences in the early onset/recent maltreatment group, F(1, 27) = .01, p > .05.
Discussion
In line with our hypotheses, maltreatment pervasiveness interacted with gender to predict diurnal cortisol and DHEA output in maltreated and nonmaltreated children. However, our findings are not completely consistent with the ACM or Kajantie and Phillips (2006) hypotheses explaining gender differences in stress system regulation. This data provides some support for Kajantie and Phillips, who predict hyporesponsitivity following trauma in females. The results for females are also consistent with the allostatic load model (Juster et al., 2011), which outlines a developmental process such that chronic stress leads to hyperresponsivity initially, but down-regulation and hyporesponsivity over time if stress continues. For example, within girls, the late onset/recent maltreatment group had higher cortisol than nonmaltreated and early onset/not recent groups. This is consistent because the late onset/recent group might have higher cortisol from the new traumatic event while girls who experienced early onset and not recent maltreatment may have started down-regulating cortisol levels, as predicted. In addition, the girls with less pervasive maltreatment had higher cortisol levels than nonmaltreated girls and girls with more pervasive maltreatment. More pervasive maltreatment could promote down-regulation of the cortisol response in females, while the less pervasive maltreatment group could be an early stage in which they are still hyperresponsive. Because the children are still in middle childhood, these results could be an intermediate step before HPA hyporesponsiveness in adulthood. For example, a study of sexually abused females indicated that although girls showed higher cortisol levels at the time that the abuse was first reported, cortisol attenuated over time (Trickett et al., 2010). By early adulthood, cortisol levels in these females were lower than in nonmaltreated females, and a similar mechanism could be operating in the girls in this group.
The findings in the boys are not as clearly explained within existing theoretical frameworks. We did not find support for the ACM’s prediction of hyporesponsivity in boys following trauma. Specifically, in the more pervasive maltreatment group, boys showed higher cortisol levels than girls, and in the less pervasive maltreatment group, boys show higher cortisol/DHEA levels than girls. Boys with less pervasive maltreatment showed higher cortisol/DHEA levels than nonmaltreated boys. In addition, boys have higher cortisol/DHEA levels than girls in the group whose maltreatment was early onset/not recent. All of these suggest hyperresponsivity for boys, which would indicate that the ACM’s hypothesis about stress responsivity in males who have experienced trauma may need revision. However, an interesting finding is the high cortisol/DHEA ratio and low DHEA levels in the boys with less pervasive maltreatment. Higher DHEA levels are often viewed as a protective factor against high cortisol, linked to better adaptation and regulation (Charney, 2004). Theoretically, a high cortisol/DHEA ratio should be associated with more health problems and poorer neuroendocrine regulation in that DHEA is not balancing out cortisol. There is no strict cutoff for cortisol/DHEA levels that indicates risk for disorder so interpretation of the levels observed in our boys with less pervasive maltreatment is not clear. However, high levels reported in depressed individuals relative to controls indicate that these boys may be at risk for future psychopathology (Goodyer, Herbert, & Altham, 1998; Young, Gallagher, & Porter, 2002). Yehuda and colleagues (2006) report that higher DHEA levels and lower cortisol/DHEA levels were found in adult males with PTSD, unlike the boys with less pervasive maltreatment in the current study, who had lower DHEA and higher cortisol/DHEA levels than girls with similar experiences. Further analyses by Yehuda et al. (2006) indicated that symptom improvement and coping predicted the higher DHEA levels and that cortisol/DHEA levels were predicted by childhood trauma severity and symptom severity. As a result, DHEA was interpreted as a defense mechanism. In the current study, boys with less pervasive maltreatment may have lower levels of DHEA because they have not experienced chronic cortisol exposure like the boys with more pervasive maltreatment. DHEA may be acting to protect the more pervasively maltreated boys but may not yet have hit a tipping point in the less pervasively maltreated children.
Although males likely experience the same mechanisms of cortisol down-regulation following chronic stress, another possible explanation for these findings is that these regulatory mechanisms may operate on a different timeline for males versus females. Puberty has been targeted as a time of HPA axis reorganization (Gunnar & Quevedo, 2007), so girls—who often experience puberty at an earlier age than boys—may undergo stress system reorganization prior to boys. As boys in this study showed greater cortisol/DHEA levels relative to girls in the early onset/not recent maltreatment group, they may experience damaging levels of cortisol for a longer period of time after maltreatment onset if they do not undergo hypothesized stress system reorganization associated with puberty until later. Because high basal cortisol levels are linked to heightened risk for negative health outcomes over time (Miller, Chen, & Parker, 2011; Taylor, Way, & Seeman, 2011), the increased levels of cortisol and the cortisol/DHEA ratio in boys might partially explain increased incidence of hypercortisolism-related disorders observed in adult men if the condition is prolonged (Heim, Ehlert, & Hellhammer, 2000; Kajantie & Phillips, 2006). On the other hand, there is evidence that hypocortisolism in children may be a marker of allostatic load and a risk factor for later depression and anxiety (Badanes, Watamura, & Hankin, 2011). Based on our results, girls with more pervasive maltreatment may be at particularly high risk for later internalizing disorders. However, as girls with more pervasive maltreatment and nonmaltreated girls did not differ in cortisol levels at this time point, it is unclear whether the more pervasively maltreated girls are at higher risk for internalizing disorders without longitudinal research.
The higher DHEA and lower cortisol/DHEA levels typically found in girls in this study compared to boys seem to suggest that females may have a protective mechanism that operates in adverse conditions. A mechanism possibly operating through DHEA would lend support to the Kajantie and Phillips evolutionary theory that women have evolved to dampen cortisol responsiveness in order to protect a potential fetus. It is possible that such mechanisms could operate even before puberty as our study and others suggest that females at high stress levels exhibit low baseline cortisol levels. This evolutionary pressure may affect non-pregnant females if the resulting alterations involve sex steroids or other mediators that operate in both stressful and non-stressful contexts.
Several mechanisms are likely operating simultaneously at the levels of the brain, endocrine glands, and the environment to produce the observed neuroendocrine differences in males and females. Sex differences may be the result of gonadal steroids, structural organization of the brain, and genomic differences (McEwen, 2001). Our finding that boys in this sample had smaller cortisol slopes across the day than girls regardless of maltreatment status indicates that biological differences between boys and girls at this age may play a role in cortisol regulation. Sexually dimorphic limbic regions in the brain as well as differences in sex steroid and corticosteroid-binding globulin levels may explain why females tend to hyposecrete cortisol following long periods of stress (Kudielka & Kirschbaum, 2005). Genes may also moderate gender and maltreatment interactions, with the stress system-related CRH receptor gene as a likely candidate (Heim et al., 2009). Several biological systems may be interacting at the neuroendocrine, immune, metabolic, and cardiovascular levels to produce the gender differences observed while increasing the allostatic load on the individual.
Psychological interpretations of social stressors are a source of individual differences in neuroendocrine levels (Miller et al., 2007), and sex differences in these psychological factors may at least partially account for our results. Perceiving an acute stressor as uncontrollable tends to increase HPA axis reactivity; however, chronic uncontrollable stress is associated with lower morning and higher evening cortisol, with higher output across the day (Miller et al., 2007). Emotions such as shame have also been related to HPA axis activity. Specifically, shame has been positively related to cortisol reactivity to acute stress, but the relationship is negative for chronic stress (Miller et al., 2007). The emotion of shame may be a mechanism by which girls who have been maltreated produce lower levels of cortisol, especially considering evidence that shame is more common in maltreated females than males (for review, see Cicchetti & Valentino, 2006). It could also be that males and females have different coping strategies or levels of social support that that buffer cortisol increases to stress. Finally, the psychiatric sequelae of maltreatment may differ between boys and girls. PTSD is commonly related to hypocortisolism while depression is more often associated with hypercortisolism and/or diurnal cortisol dysregulation (Yehuda & Seckl, 2011). Variations in the presence of psychiatric disorders or other psychological factors in boys and girls may partially explain these results.
These findings may be particularly relevant to the study of adult psychopathology as they indicate that stressful early experiences may produce differential neuroendocrine profiles in males versus females. As cortisol, DHEA, and the ratio between the two have been studied as risk and protective factors, early differences in response to experience may help to predict those at risk for onset of psychopathology in order to target individuals for preventative services. Gender differences may also point researchers to sex hormones that contribute to onset and maintenance of psychopathology. For instance, future studies could examine estrogen as a possible contributor to hypocortisolemia in response to trauma in females.
There were limitations to this study that must be addressed. First, the study design was not longitudinal so we are unable to show whether these neuroendocrine patterns existed earlier in childhood and whether they will continue through adolescence and adulthood. Boys and girls may exhibit similarities or differences in neuroendocrine profiles depending on age, time until puberty, and time since maltreatment onset. The cross-sectional nature of the study does not allow for claims of causation by particular biological and/or psychological factors. Due to the careful matching of the control group on critical SES and family variables, though, we are confident that these results are indeed sequelae of child maltreatment.
Second, it is challenging to quantify maltreatment variables because we are unable to assess the child’s interpretation of the meaning of their experience. However, due to the thorough nature of the maltreatment classification coding, we are confident that the objective constructs measured are valid. Future studies will have to examine children’s experiences prospectively to assess both the objective and subjective experiences of the child. Third, we measured diurnal cortisol and DHEA output, but we did not assess reactivity to a stressor. As a result, we can only report basal neuroendocrine levels. There may be gender and maltreatment interactions in predicting cortisol reactivity that would be important to consider. Cortisol reactivity in maltreated and nonmaltreated boys and girls should be examined to approach this component of HPA axis activity. Given the frequent disorganization in the home life of many of the families in our study, we did not have a valid means to obtain specific wake up times, and thus were not able to control for exact time of awakening in our analyses. Finally, we did not assess pubertal status in these 8- to 10-year-old children so we are unable to control for the effect of early pubertal changes on neuroendocrine outcomes.
The results of the current study provide significant theoretical and practical implications. A consistent epidemiological finding is that there are significant gender differences in the rates of mental and physical illness. For example, women are more likely to suffer from depression and anxiety, while men are at higher risk for antisocial behavior and substance abuse (Kudielka & Kirschbaum, 2005). Although cardiovascular disease, arteriosclerosis, and infectious disease are more common in men, disorders such as asthma, rheumatoid arthritis, chronic fatigue syndrome, and fibromyalgia, which have been linked to hypocortisolism, are more prevalent in women (Heim, Ehlert, & Hellhammer, 2000; Kajantie & Phillips, 2006). Because females experience higher rates of hypocortisolism-related disorders, it could be that women are more likely to experience a persistent diminution of cortisol in response to chronic stress and thus develop specific stress-related disorders (Heim, Ehlert, & Hellhammer, 2000). Our findings partially support this hypothesis and may help explain the early origins of these epidemiological discrepancies if gender differences in neuroendocrine regulation are indeed a contributing factor.
Explanations of sex differences may strengthen theories of neuroendocrine activity in response to stress. In addition, researchers can use these results to better understand how maltreatment affects children biologically so that interventions can better target and treat individuals. Recent research has demonstrated the ability of psychosocial interventions to prevent cortisol dysregulation in at-risk children as young as infancy (e.g., Cicchetti et al., 2011). The effectiveness of these interventions may be improved with greater knowledge of HPA axis moderators, including gender and the subtype, severity, and chronicity of maltreatment. With better prevention and treatment of disorders related to maltreatment, a significant amount of stress on the child, family, and community will be lessened, and the harmful mental and physical sequelae of maltreatment will be reduced.
Table 3.
Maltreatment variables by sex
| Variable | Males | Females | % Males | % Females |
|---|---|---|---|---|
| Sum of subtypes: 2+ subtypes | 38 | 27 | 53.5 | 50 |
| 1 subtype | 33 | 27 | 46.5 | 50 |
| Sum of severity: More severe | 21 | 11 | 29.6 | 20.4 |
| Less severe | 50 | 43 | 70.4 | 79.6 |
| Developmental Periods: 2+ periods | 33 | 21 | 46.5 | 38.9 |
| 1 period | 38 | 33 | 53.5 | 61.1 |
| Early Onset/Not Recent | 33 | 25 | 46.5 | 46.3 |
| Early Onset/Recent | 22 | 12 | 31.0 | 22.2 |
| Late Onset/Recent | 16 | 17 | 22.5 | 31.5 |
| Experienced neglect | 61 | 41 | 80.3 | 71.9 |
| Experienced emotional abuse | 36 | 29 | 47.4 | 50.9 |
| Experienced physical abuse | 19 | 12 | 25 | 21.1 |
| Experienced sexual abuse | 7 | 5 | 9.2 | 8.8 |
Note: Based on maltreated group only. The variable % Males represents the percent of males in each category, and % Females represents the percent of females in each category.
Acknowledgments
Role of the Funding Source
Study sponsors provided financial support but did not have a role in data collection, analysis, or interpretation. The decision to submit the paper for publication was the sole decision of the corresponding authors.
This research was supported by funding from the National Institute of Mental Health (MH083979) and the Spunk Fund, Inc. In addition, an NIMH training grant (T32MH015755, Dante Cicchetti, PI) supported Jenalee Doom.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to report.
Contributors
Jenalee Doom conceptualized the ideas for this study, conducted statistical analyses, and wrote the manuscript. Dante Cicchetti wrote the grants that funded the study, planned data collection, and helped the first author with manuscript preparation. Fred Rogosch and Melissa Dackis helped with data cleanup and statistical analyses. All authors have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Achenbach T. Manual for the Teacher Report Form and 1991 profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- 2.Badanes LS, Watamura SE, Hankin BL. Hypocortisolism as a potential marker of allostatic load in children: associations with family risk and internalizing disorders. Dev Psychopathol. 2011;23:881–896. doi: 10.1017/S095457941100037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett D, Manly JT, Cicchetti D. Defining child maltreatment: The interface between policy and research. In: Cicchetti D, Toth SL, editors. Child Abuse, Child Development, and Social Policy. Abex; Norwood, NJ: 1993. pp. 7–73. [Google Scholar]
- 4.Bicanic IAE, Postma RM, Sinnema G, De Roos C, Olff M, Van Wesel F, Van de Putte EM. Salivary cortisol and dehydroepiandrosterone sulfate in adolescent rape victims with post traumatic stress disorder. Psychoneuroendocrino. 2012 doi: 10.1016/j.psyneuen.2012.06.015. Available online 4 August 2012. [DOI] [PubMed] [Google Scholar]
- 5.Bolger KE, Patterson CJ, Kupersmidt JB. Peer relationships and self-esteem among children who have been maltreated. Child Dev. 1998;69:1171–1197. [PubMed] [Google Scholar]
- 6.Bremner D, Vermetten E, Kelley ME. Cortisol, dehydroepiandrosterone, and estradiol measured over 24 hours in women with childhood sexual abuse-related posttraumatic stress disorder. J Nerv Ment Dis. 2007;195:919–927. doi: 10.1097/NMD.0b013e3181594ca0. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiat. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 9.Cicchetti D, Manly JT. A personal perspective on conducting research with maltreating families: Problems and solutions. In: Brody G, Sigel I, editors. Methods of Family Research: Families at Risk. Vol. 2. Erlbaum; Hillsdale, NJ: 1990. pp. 87–133. [Google Scholar]
- 10.Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Dev Psychopathol. 2001a;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- 11.Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Dev Psychopathol. 2001b;13:783–804. [PubMed] [Google Scholar]
- 12.Cicchetti D, Rogosch FA. Personality, adrenal steroid hormones, and resilience in maltreated children: A multilevel perspective. Dev Psychopathol. 2007;19:787–809. doi: 10.1017/S0954579407000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child Dev. 2010;81:252–269. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cicchetti D, Rogosch FA, Toth SL, Sturge-Apple ML. Normalizing the development of cortisol regulation in maltreated infants through preventive interventions. Dev Psychopathol. 2011;23:789–800. doi: 10.1017/S0954579411000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cicchetti D, Toth SL, Manly JT. Maternal Maltreatment Classification Interview. 2003 Unpublished manuscript. [Google Scholar]
- 16.Cicchetti D, Valentino K. An ecological transactional perspective on child maltreatment: Failure of the average expectable environment and its influence upon child development. In: Cicchetti D, Cohen J, editors. Developmental Psychopathology. 2. Vol. 3. Wiley; New York: 2006. pp. 129–201. Risk, Disorder, and Adaptation. [Google Scholar]
- 17.De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Ryan ND. Developmental traumatology part I: Biological stress systems. Biol Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- 18.De Bellis M, Chrousos G, Dorn L, Burke L, Helmers K, Kling M, Trickett P, Putman F. Hypothalamic–pituitary–adrenal axis dysregulation in sexually abused girls. J Clin Endocrinol Metab. 1994;78:249–255. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- 19.Del Giudice M, Ellis BJ, Shirtcliff EA. The adaptive calibration model of stress responsivity. Neuroscience and Biobehavioral Reviews. 2011;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Goodyer IM, Herbert J, Altham PME. Adrenal steroid secretion and major depression in 8- to 16-year-olds, III: influence of cortisol/DHEA ratio at presentation on subsequent rates of disappointing life events and persistent major depression. Psychol Med. 1998;28:265–273. doi: 10.1017/s0033291797006314. [DOI] [PubMed] [Google Scholar]
- 22.Goodyer IM, Park RJ, Netherton CM, Herbert J. Possible role of cortisol and dehydroepiandrosterone in human development and psychopathology. British J Psychiat. 2001;179:243–249. doi: 10.1192/bjp.179.3.243. [DOI] [PubMed] [Google Scholar]
- 23.Granger DA, Schwartz EB, Booth A, Curran M, Zakaria D. Assessing dehydroepiandrosterone in saliva: A simple radioimmunoassay for use in studies of children, adolescents, and adults. Psychoneuroendocrino. 1999;24:567–579. doi: 10.1016/s0306-4530(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 24.Gunnar MR, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–73. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- 25.Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, Nemeroff CB, et al. Effect of childhood trauma on adult depression and neuroendocrine function: Sex-specific moderation by CRH receptor 1 gene. Frontiers in Behavioral Neuroscience. 2009;3:41. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrino. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 27.Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biol Psychiat. 2008;63:398–405. doi: 10.1016/j.biopsych.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depress Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- 29.Jessop DS, Turner-Cobb JM. Measurement and meaning of salivary cortisol: a focus on health and disease in children. Stress. 2008;11:1–14. doi: 10.1080/10253890701365527. [DOI] [PubMed] [Google Scholar]
- 30.Juster RP, Bizik G, Picard M, Arsenault-Lapierre G, Sindi S, Trepanier L, et al. A transdisciplinary perspective of chronic stress in relation to psychopathology throughout life span development. Dev Psychopathol. 2011;23:725–776. doi: 10.1017/S0954579411000289. [DOI] [PubMed] [Google Scholar]
- 31.Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrino. 2006;31:151–78. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 33.MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, et al. Cortisol response to stress in female youths exposed to childhood maltreatment: Results of the youth mood project. Biol Psychiat. 2009;66:62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manly JT. Advances in research definitions of child maltreatment. Child Abuse Neglect. 2005;29:425–439. doi: 10.1016/j.chiabu.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Manly JT, Kim JE, Rogosch FA, Cicchetti D. Dimensions of child maltreatment and children’s adjustment: contributions of developmental timing and subtype. Dev Psychopathol. 2001;13:759–782. [PubMed] [Google Scholar]
- 36.McCrory E, DeBrito SA, Viding E. The impact of childhood maltreatment: a review of neurobiological and genetic factors. Frontiers in Child and Neurodevelopmental Psychiatry. 2011;2:1–14. doi: 10.3389/fpsyt.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McEwen BS. Estrogens effects on the brain: Multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- 38.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 40.Netherton C, Goodyer I, Tamplin A, Herbert J. Salivary cortisol and dehydroepiandrosterone in relation to puberty and gender. Psychoneuroendocrino. 2004;29:125–40. doi: 10.1016/s0306-4530(02)00150-6. [DOI] [PubMed] [Google Scholar]
- 41.Ouellet-Morin I, Odgers CL, Danese A, Bowes L, Shakoor S, Papadopoulos AS, Arseneault L. Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biol Psychiatry. 2011;70:1016–1023. doi: 10.1016/j.biopsych.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sedlak AJ, Mettenburg J, Basena M, Petta I, McPherson K, Greene A, et al. Fourth National Incidence Study of Child Abuse and Neglect (NIS–4): Report to Congress, executive summary. Washington, DC: U.S. Department of Health and Human Services, Administration for Children and Families; 2010. [Google Scholar]
- 43.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 44.Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Horm Behav. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 45.Taylor SE, Way BM, Seeman TE. Early adversity and adult health outcomes. Dev Psychopathol. 2011;23:939–954. doi: 10.1017/S0954579411000411. [DOI] [PubMed] [Google Scholar]
- 46.Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Dev Psychopathol. 2010;22:165–175. doi: 10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Goozen SHM, Fairchild G. How can the study of biological processes help design new interventions for children with severe antisocial behavior? Dev Psychopathol. 2008;20:941–973. doi: 10.1017/S095457940800045X. [DOI] [PubMed] [Google Scholar]
- 48.Van Voorhees E, Scarpa A. The effects of child maltreatment on the hypothalamic– pituitary–adrenal axis. Trauma Violence Abuse. 2004;5:333–352. doi: 10.1177/1524838004269486. [DOI] [PubMed] [Google Scholar]
- 49.Yehuda R, Brand SR, Golier JA, Yang RK. Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta Psychiatr Scand. 2006;114:187–193. doi: 10.1111/j.1600-0447.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- 50.Yehuda R, Seckl J. Stress-related psychiatric disorders with low cortisol levels: A metabolic hypothesis. Endocrinology. 2011;152:4496–4503. doi: 10.1210/en.2011-1218. [DOI] [PubMed] [Google Scholar]
- 51.Young AH, Gallagher P, Porter RJ. Elevation of the cortisol- dehydroepiandrosterone ratio in drug-free depressed patients. Am J Psychiat. 2002;159:1237–1239. doi: 10.1176/appi.ajp.159.7.1237. [DOI] [PubMed] [Google Scholar]