Fig. 6. Label-free MST for quantification of GPCR A2AR ligand binding.
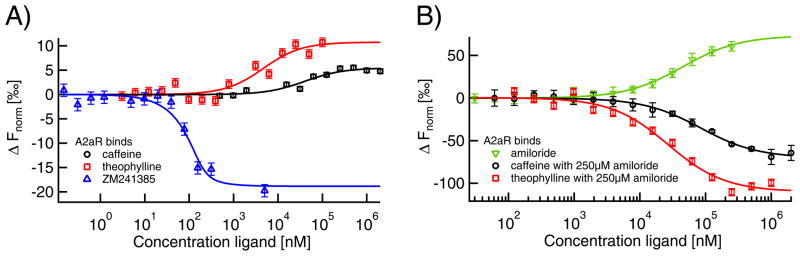
A) Binding of the orthosteric anatagonists caffeine (KD=40±17 μM; black circles), theophylline (KD=5±2 μM; red squares) and ZM241385 (KD≤43 nM; blue triangles) to A2aR induces a comparably small change in thermophoretic mobility. B) In contrast, amiloride-binding (KD=52±7 μM; green inverted triangles) leads to a much larger MST signal amplitude, thus indicating conformational changes upon binding. Comparable signal amplitudes were obtained for the binding of caffeine and theophylline in presence of saturating amiloride concentrations, where the apparent affinities were decreased to 84±10 μM for caffeine and 27±6 μM for theophylline.
