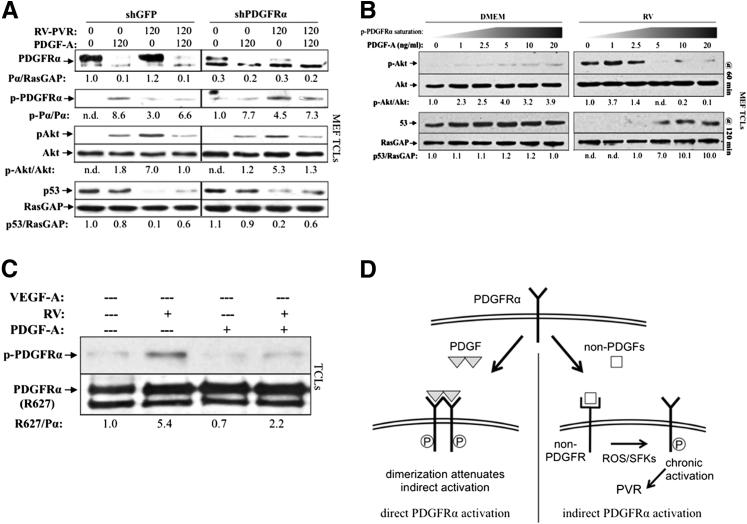
Figure 3.
PDGF-mediated dimerization attenuated indirect activation of PDGFRα. A: Reduction of PDGFRα levels by shRNA did not attenuate indirect PDGFRα signaling. Lentiviruses were used to stably express shRNAs directed against green fluorescent protein (GFP; shGFP) or PDGFRα (shPDGFRα) in MEFs; shPDGFRα MEFs expressed approximately 70% less receptor than control shGFP MEFs. Cells were then starved and treated with 400 μL RV-PVR and/or 20 ng/mL PDGF-A, as indicated for 120 minutes at 37°C, then lysed and subjected to Western blot analysis using the indicated antibodies. Both immature and mature PDGFRα band intensities were normalized to RasGAP levels. These findings indicate that no more than 30% of the total pool of PDGFRα was required to trigger signaling events in response to RV-PVR. B: Near-confluent MEFs were serum starved overnight and treated with 400 μL DMEM or RV supplemented with an increasing concentration of PDGF-A. Cells were treated in parallel for 60 and 120 minutes, after which time they were harvested and TCLs were subjected to Western blot analysis using the indicated antibodies. Prolonged Akt phosphorylation and reduction in p53 levels (indicators of indirectly activated PDGFRα)39 were attenuated at saturating doses of PDGF-A, suggesting that PDGF-induced dimerization is a key component of PDGF-mediated attenuation of vitreous-driven indirect signaling of PDGFRα. C: R627 cells were serum starved overnight and either left alone or pre-incubated with 10 ng/mL PDGF-A for 30 minutes at 4°C (to ensure complete receptor dimerization on the cell surface), followed by treatment with or without 400 μL RV for 10 minutes at 4°C. After treatment, cells were lysed and subjected to Western blot analysis using anti–phospho-PDGFRα, followed by anti-pan PDGFRα, and normalized relative to the untreated control. Although indirect activation of PDGFRα still occurred at 4°C, receptor activation was approximately 2.5-fold lower compared with indirect receptor activation at 37°C (Supplemental Figure S4), demonstrating a correlation between dimerization and reduced capacity to undergo indirect activation, further suggesting that PDGF diminishes non–PDGF-mediated activation of PDGFRα by dimerizing PDGFRαs. D: Schematic showing the details and consequences of direct and indirect activation of PDGFRα. ROS, reactive oxygen species; SFK, Src-family kinases.