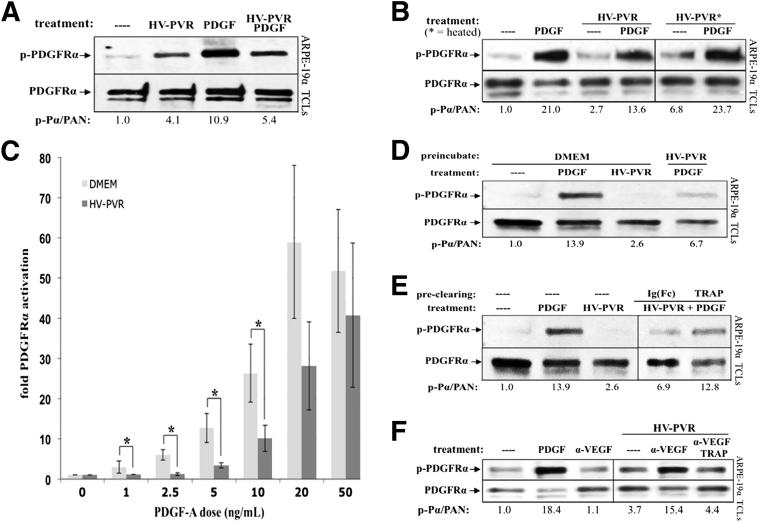
Figure 4.
PDGF-dependent activation of PDGFRα was inhibited by VEGF-A, a heat-labile, PDGFRα-associated agent in human PVR vitreous. A: Patient PVR vitreous contains inhibitory activity against PDGF-mediated PDGFRα activation. ARPE-19α cells were grown to near confluence, serum starved overnight, and then treated with serum-free medium alone (—), 200 μL human patient PVR vitreous (HV-PVR), 10 ng/mL PDGF-A, or both HV-PVR and PDGF-A for 5 minutes at 37°C. Cells were lysed and subjected to anti–phospho-PDGFRα (p-PDGFRα) and then anti-PDGFRα Western blot analysis. The p-PDGFRα immunoblot signal was normalized to total PDGFRα (PAN) and is presented as fold induction over the non-stimulated control. Blots shown are representative of three independent experiments. HV-PVR reduced PDGF-mediated activation of PDGFRα by approximately 50%, suggesting that patient PVR vitreous contains an inhibitor of PDGF-mediated PDGFRα activation. B: Inhibitory activity in human patient PVR vitreous (HV-PVR) is labile to heat. In a manner similar to A, cells were starved and treated with serum-free medium without treatment (—), 200 μL HV-PVR, 10 ng/mL PDGF-A, or both HV-PVR and PDGF-A; some HV-PVR treatments were first heat treated to 90°C for 5 minutes and then rapidly cooled on ice. Cells were treated for 5 minutes at 37°C and lysed, and the resulting TCLs were subjected to the same Western blot analysis as in A. Although PDGF-A largely survived the heat treatment, nearly all PDGF-inhibitory activity was eliminated from HV-PVR. Moreover, there were enough endogenous PDGFs in heat-treated HV-PVR to elicit a 3.5-fold activation of PDGFRα (over the non-stimulated control). Thus, heat treatment unmasked the ability of HV-PVR to activate PDGFRα, suggesting the presence of a heat-labile inhibitor that blocks vitreal PDGFs from functioning. C: HV-PVR–mediated inhibition of PDGF-dependent PDGFRα could be overcome by increasing the concentration of PDGF. Cells were cultured and starved as described in A. The indicated amount of PDGF-A was added to either 200 μL DMEM or HV-PVR and then used to treat cells for 5 minutes at 37°C. Cells were lysed and subjected to Western blot analysis, and the results were quantified as in Figure 1. Results from three independent experiments revealed that HV-PVR significantly inhibited PDGFRα phosphorylation at low doses of PDGF-A: 1, 2.5, 5, and 10 ng/mL. ∗P < 0.05 using a paired t-test. D: Cells preconditioned with patient PVR vitreous became resistant to subsequent treatment with PDGF-A. Cells were cultured and starved, as described in A, then pre-incubated for 15 minutes at 37°C with either DMEM or 200 μL HV-PVR. After incubation, the media/vitreous was removed and cells were extensively washed with PBS, after which they were treated with serum-free medium alone (—) or 10 ng/mL PDGF-A for 10 minutes at 37°C. Cells were subsequently lysed, and the resulting TCLs were subjected to Western blot analysis with the indicated antibodies. These data suggest that the inhibitor(s) present in HV-PVR acted at the level of cells. E: Preclearing HV-PVR vitreous with PDGF TRAP significantly reduced its ability to inhibit PDGFRα activation by exogenously added PDGF. HV-PVR (200 μL) was not manipulated or precleared with 2 μmol/L PDGF TRAP or an equimolar amount of a control IgG-Fc fragment (IgG-Fc). These clarified samples were then tested for their ability to block PDGFRα activation by exogenously added PDGF-A. To this end, cells were treated with clarified vitreous and 10 ng/mL PDGF-A for 10 minutes at 37°C. Serum-free media without treatment (—) and 10 ng/mL PDGF-A alone were used as negative and positive controls, respectively. Cells were lysed, and the resulting TCLs were subjected to the same Western blot analysis as used in A. The ability of PDGF TRAP to reduce PDGF-inhibitory activity from HV-PVR suggests that this inhibitor can associate with the extracellular domain of PDGFRα. F: Neutralizing VEGF-A in human PVR vitreous with ranibizumab enabled vitreal PDGFs to activate PDGFRα. Cells were serum starved overnight and either lysed immediately (—) or treated for 10 minutes at 37°C with 10 ng/mL PDGF-A, 10 μg/mL α-VEGF, or 200 μL HV-PVR supplemented with 10 μg/mL nonimmune IgG, 10 μg/mL α-VEGF, or a combination of 10 μg/mL α-VEGF and 2 μmol/L PDGF TRAP. After treatment, cells were lysed and the resulting TCLs were subjected to Western blot analysis using the indicated antibodies and quantified. Ratios representing normalized band intensities are shown under each immunoblot. Blots shown are representative of three independent experiments. These results show that neutralizing VEGF-A significantly enhanced the ability of vitreal PDGFs to activate PDGFRα.