Abstract
A near-term female neonate with total serum bilirubin levels not exceeding the exchange transfusion level with hypoalbuminaemia developed abnormal movements while recovering from sepsis. Investigations showed an evidence of kernicterus on brain imaging and bilateral auditory neuropathic changes.
Background
Neonatal jaundice leading to acute bilirubin encephalopathy (ABE) is the recognised cause of bilirubin-induced neurological dysfunction, which is manifested as kernicterus, athetoid cerebral palsy, auditory neuropathy (AN), ocular movement disorders and dental enamel dysplasia. Although rare, the principal aim of treating hyperbilirubinaemia is to avoid these neurological sequelae.1 Recent National Institute for Health and Clinical Excellence (NICE) guidelines aim to identify neonates at risk of ABE appropriately while also avoiding unnecessary treatment in the low-risk group.2
This case highlights that hypoalbuminaemia and sepsis are major risk factors for kernicterus even when serum bilirubin levels are below nationally agreed treatment threshold. The efficacy of total serum bilirubin (TSB) versus unbound (free) bilirubin levels together with bilirubin/albumin ratios as a means of identifying neonates at risk of kernicterus are reviewed.
Case presentation
A 36+6-week gestation Caucasian female infant was born to a primigravid woman by instrumental vaginal delivery. Antenatal imaging and serology were normal and there was no family history of abnormal movement. There had been prolonged (greater than 24 h) prelabour rupture of membranes and adequate intrapartum antibiotic cover was given. The infant was born in good condition with Apgar scores of 8 and 10 at 1 and 5 min, respectively, with a birth weight of 2.826 kg. She required admission to the neonatal unit at 3 h of life with respiratory symptoms. On admission, a partial septic screen was performed and intravenous antibiotics were given. She was kept nil by mouth and started on intravenous fluids. Continuous positive airway pressure support was given, which was changed to synchronised intermittent mandatory ventilation (SIMV) at 8 h after birth owing to worsening respiratory acidosis and chest x-ray changes consistent with congenital pneumonia. Initial baseline blood tests were all within normal limits. Following the start of SIMV, subsequent capillary blood gases were also normal.
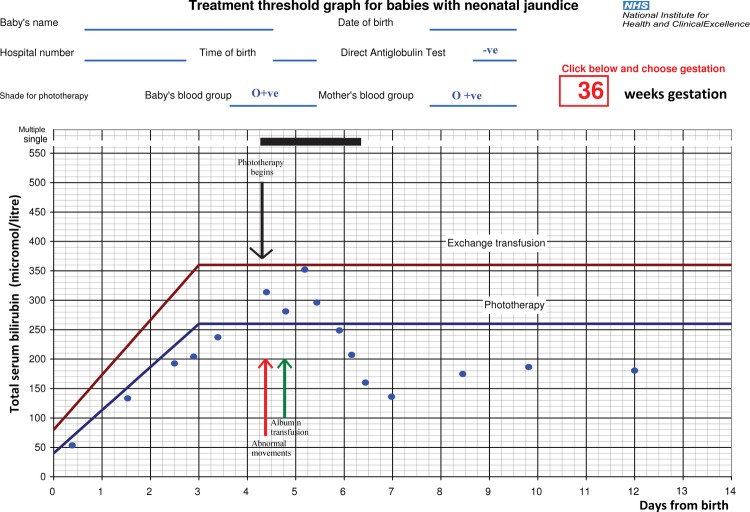
There was a rise in C reactive protein from an initial value of 1 mg/l on day 1 to 86 mg/l on day 3 (figure 1). The maternal high vaginal swab grew group B Streptococcus and treatment was given with benzylpenicillin and gentamicin. A lumbar puncture did not show evidence of meningitis, but the penicillin dose was doubled. Clinical examination showed an evidence of mild jaundice with the TSB level below the phototherapy treatment threshold according to the UK NICE guideline2 (see figure 2).
Figure 1.
A graph showing the biochemical markers measured on each day. Each arrow is annotated with events that occurred at each time such as the level of total serum bilirubin when phototherapy began, when abnormal movements were noticed as well as when albumin infusion was indicated.
Figure 2.
National Institute for Health and Clinical Excellence (NICE) jaundice threshold graph for 36 weeks gestation with total serum bilirubin (TSB) levels plotted at appropriate times. Phototherapy was started at approximately 102 h after birth, as seen above the TSB levels never exceeded the exchange transfusion treatment threshold.
On days 4–5, there were low serum albumin levels 17–19 g/l. At 102 h after birth there was a further rise in TSB levels above phototherapy threshold but below the exchange threshold with less than 10% conjugated fraction. All other haematological and biochemical inflammatory markers were improving and the blood and cerebrospinal fluid (CSF) cultures remained negative. At no point during treatment did the TSB level exceed the NICE recommended exchange transfusion threshold2; the neonate was started on double phototherapy; however, 5 h into phototherapy treatment on day 4 the infant was noted to have abnormal movements with opisthotonus and intermittent dystonic movements in the upper limbs. An infusion of albumin was given in view of the persistently low serum albumin level. Enteral feeds with maternal expressed breast milk were given at 120 h after birth.
Days 6–10, phototherapy was continued with reducing TSB levels. The abnormal movements persisted. Subsequent metabolic, genetic, haematological and biochemical investigations were all negative. Electroencephalography (EEG) and cranial ultrasound scan results were normal. An MRI of the brain showed bilateral increased signal changes in the globus pallidus and subthalamic nuclei. There were no other abnormalities. The findings support the diagnosis of kernicterus. Auditory brainstem response (ABR) and otoacoustic emission tests were carried out. These showed evidence of an AN spectrum disorder in both ears.
Investigations
Routine measurement of haematological, biochemical and inflammatory markers (see figure 1and 2 for results of C reactive protein, serum bilirubin and serum albumin).
Metabolic investigations including urine organic acids, plasma amino acids, acylcarnitine and serum lactate were normal.
Normal chromosomes.
Glucose, lactate, protein and cell count levels in the CSF were normal.
Negative blood and CSF culture.
Cranial ultrasound scan and EEG were normal.
MRI showed bilateral increased signal changes in the globus pallidus and subthalamic nuclei. No other abnormalities were found. The findings support the diagnosis of kernicterus.
ABR and otoacoustic emission tests supported the diagnosis of bilateral auditory neuropathic changes.
Differential diagnosis
Inborn error of metabolism
Meningitis/encephalopathy
Cerebrovascular ischaemic event
Treatment
Treatment of sepsis with appropriate antibiotics.
Treatment of hyperbilirubinaemia with double phototherapy.
Treatment of hypoalbuminaemia with an albumin infusion.
Outcome and follow-up
She had a full course of antibiotics, with complete resolution of her sepsis and jaundice and following establishment of breastfeeding and was discharged on day 17. She remains under neonatal developmental and audiological follow-up. Follow-up at 8 months showed findings suggestive of early neurodevelopmental delay and choreiform movements consistent with kernicterus.
Discussion
Jaundice requiring phototherapy is very common, kernicterus on the other hand is very rare with most neonatal tertiary units seeing approximately 1 case every 10 years; cases of low bilirubin kernicterus are extremely rare.
Systemic bacterial infection (especially, septicaemia and urinary tract infections) may increase the severity of jaundice after 24 h of life. While not all causes of jaundice are risk factors for ABE, sepsis on its own has been identified as a major risk factor for ABE in neonates. A study comparing 18 mature infants with confirmed sepsis against a control group suggested that even though there were no significant differences in the unconjugated bilirubin (UCB) concentration and plasma pH in both groups, there was a slight increased risk of bilirubin encephalopathy in the group with sepsis.3 This is thought to be because of a significant reduction in the albumin concentration, as well as a reduction in albumin binding capacity because of sepsis.3
Studies have also shown that the amount of UCB in and around the central nervous system depends on the amount of UCB crossing the blood–brain barrier (BBB) and not necessarily the TSB.4 The rate of absorption of UCB is determined by the permeability of the BBB.4 Therefore, factors that affect the BBB permeability alter the exposure of the brain cells to UCB; these factors include hyperosmolality, severe asphyxia, increased cerebral venous return, increased cerebral blood flow owing to hypercarbia and increased bilirubin–albumin dissociation because of altered sepsis.5 Most circulating UCB is bound to plasma protein which does not cross the BBB or cell membranes at all. However where there are low levels of albumin, inadequate binding of UCB leads to higher levels of unbound UCB able to cross the BBB. Consequently low serum albumin levels increase the risk of bilirubin neurotoxicity.1 The level at which this becomes critical is unknown.
Ahlfors et al1 proposed that measurement of free bilirubin or bilirubin/albumin ratio should be incorporated into clinical practice when assessing neonates at risk of bilirubin neurotoxicity. At present the UK guideline on neonatal jaundice does not recommend the use of the bilirubin/albumin ratio in the assessment of risk for kernicterus. Awareness of the increased risk of kernicterus associated with sepsis and hypoalbuniaemia is highlighted by this case.
Learning points.
This highlights the increased risk of acute bilirubin encephalopathy in neonates with low total serum bilirubin levels following sepsis especially where there are low albumin levels in addition.
A reduction in albumin binding sites leads to increased bilirubin dissociation and increased free unconjugated bilirubin (UCB) can readily cross the blood–brain barrier.
Free (UCB) bilirubin levels are difficult to measure and routine measurement of bilirubin/albumin ratios is not part of the current National Institute for Health and Clinical Excellence (NICE) guidance on the management of neonatal hyperbilirubinaemia.
Whether the routine use of the bilirubin/albumin ratio would help reduce the incidence of low bilirubin kernicterus remains to be proven.
Footnotes
Contributors: The article was written by YO under the supervision of educational supervisor AJE, he identified the case as one that needs to be reiterated and also helped with proofreading, editing and approval for the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ahlfors CE, Wennberg RP, Ostrow JD, et al. Unbound (Free) bilirubin: improving the paradigm for evaluating neonatal jaundice. Clin Chem 2009;2013:1288–99 [DOI] [PubMed] [Google Scholar]
- 2.2010. NICE neonatal Jaundice Clinical Guidelines. http://www.nice.org.uk/nicemedia/live/12986/48678/48678.pdf (accessed 12 May 2012).
- 3.Ebbesen F, Knudsen AA. The risk of bilirubin encephalopathy, as estimated by plasma parameters, in neonates strongly suspected of having sepsis. Acta Paediatr 1993;2013:26–9 [DOI] [PubMed] [Google Scholar]
- 4.Wennberg RP, Ahlfors CE, Bhutani VK, et al. Toward understanding kernicterus: a challenge to improve the management of jaundiced newborns. Pediatrics 2006;2013:474–83j.c [DOI] [PubMed] [Google Scholar]
- 5.Hulzebos CV, Van Imhoff DE, Bos AF. Usefulness of the bilirubin/albumin ratio for predicting bilirubin-induced neurotoxicity in premature infants. Arch Dis Child Fetal Neonatal Ed 2008;2013:384–8 [DOI] [PubMed] [Google Scholar]