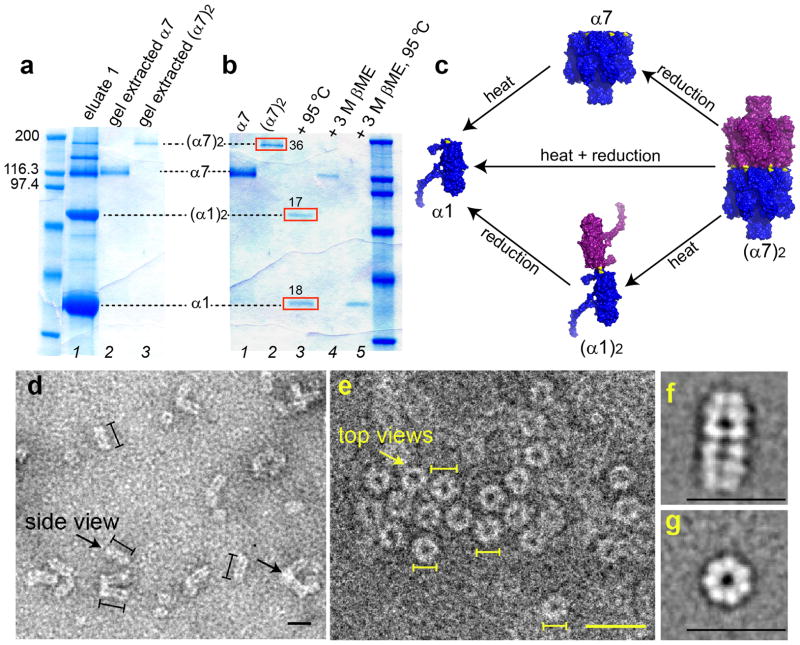
Figure 2. Expression, purification and structural characterization of (α7)2.
(a) SDS-polyacrylamide gel showing the purification of (α7)2. The Ni2+-NTA purified αHL mutant K237C/D8H6 contained a mixture of the monomer α1, the monomer dimer (α1)2, the heptamer α7, and the heptamer dimer (α7)2. α7 and (α7)2 were purified (lanes 2 and 3) by extraction from a preparative gel (Supplementary Methods). (b) Heating (α7)2 at 95°C for 10min yielded α1 and (α1)2 (red boxes, lane 3) with relative band intensities of 18 and 17 absorbance units, derived from 36 absorbance units of (α7)2 (red box, lane 2). To determine whether the (α7)2 was composed of α7 units linked by disulfide bonds, a sample was treated with 3M βME for 15min at 25°C, which indeed produced α7 (lane 4). Upon heating at 95°C, followed by reduction with 3M βME for 15min at 25°C, (α7)2 dissociated into α1 (lane 5). (c) A model for the structural composition and dissociation of (α7)2 based on the electrophoresis studies. (d) Electron micrographs after uranyl acetate staining showing side views (left arrow) of (α7)2 as elongated particles of length 19.6±0.2nm (mean±s.e.m., n=268) and width 8.4±0.1nm (mean±s.e.m.; n=96), which correspond to the length (~20nm) and width (~8.5nm) of (α7)2 derived from a molecular model (Fig. 1b). (α7)2 particles were also observed aggregated longitudinally in clusters of three to four molecules most probably through the hydrophobic β barrel domains (right arrow). (e) Electron micrographs showing ring views of (α7)2. The average diameter of the particles is 7.8±0.1nm (mean±s.e.m., n=131). Scale bars in d, e, 20nm. Lines drawn next to single particles (d: black; e: yellow) denote the boundaries used to measure lengths. (f) Class average of 309 single particles showing the side view. (g) Class average of 224 single particles showing the ring view.