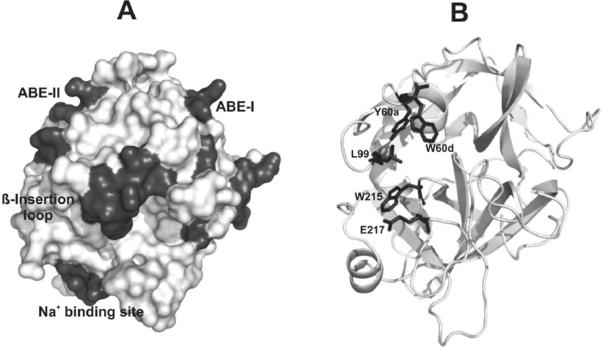
Figure 1. X-ray crystal structures highlighting major regions of thrombin.
(A) A contour representation of thrombin (PDB entry 1PPB) is displayed. The β- or 60-insertion loop hangs over the serine protease active site and restricts substrate access. Anion binding exosite II is located to the left of the active site whereas anion binding exosite I is located to the right. The sodium binding site serves an allosteric role. (B) The same thrombin structure is displayed in cartoon format. The five residues that are mutated are highlighted in stick form. W60d andY60a are members of the 60-insertion loop and L99 is part of the S2 pocket. W215 serves as an important platform for substrates. E217 participates in the Na+ allosteric network and stabilizes the S1 site.