Figure 6. Scheme interrelating repetitive daily ethanol-induced changes in AQP4, PLA2 families, ROS, and PARP-1 to proposed associations with neuroinflammation and neurodamage.
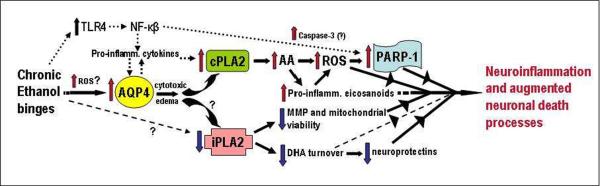
Assuming that transcriptional rather than turnover alterations are principally responsible for changes in levels, AQP4 elevations due to repeated daily ethanol intoxication, possibly stimulated by early ROS bursts, could potentiate pro-inflammatory cytokines and trigger increased cPLA2 levels/activity and AA mobilization. AA is known to generate ROS nonenzymatically and via eicosanoid biosynthesis. Pro-inflammatory eicosanoids such as leukotrienes could also have receptor-mediated neuroinflammatory effects. PARP-1 elevations could be dependent on ROS, but also on increased NF-kappaB activity. The NF-kappaB pathway is stimulated by toll-like receptor 4 (TLR4), which is known to be activated as well by chronic ethanol binges. How ethanol might decrease iPLA2 levels is not evident (question marks); nevertheless, significant loss of the enzyme may lead to decreased mitochondrial membrane permeability (MMP) and viability and reduced docosahexaenoic acid (DHA) turnover. Diminished DHA could lead to less pro-survival effects, including neuroprotectin (e.g., NPD-1) formation. See text for supporting references.
Tajuddin et al.
