Abstract
Photobiological H2 production is an attractive option for renewable solar fuels. Sulfur-deprived cells of Chlamydomonas reinhardtii have been shown to produce hydrogen with the highest efficiency among photobiological systems. We have investigated the photosynthetic reactions during sulfur deprivation and H2 production in the wild-type and state transition mutant 6 (Stm6) mutant of Chlamydomonas reinhardtii. The incubation period (130 h) was dissected into different phases, and changes in the amount and functional status of photosystem II (PSII) were investigated in vivo by electron paramagnetic resonance spectroscopy and variable fluorescence measurements. In the wild type it was found that the amount of PSII is decreased to 25% of the original level; the electron transport from PSII was completely blocked during the anaerobic phase preceding H2 formation. This block was released during the H2 production phase, indicating that the hydrogenase withdraws electrons from the plastoquinone pool. This partly removes the block in PSII electron transport, thereby permitting electron flow from water oxidation to hydrogenase. In the Stm6 mutant, which has higher respiration and H2 evolution than the wild type, PSII was analogously but much less affected. The addition of the PSII inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea revealed that ∼80% of the H2 production was inhibited in both strains. We conclude that (i) at least in the earlier stages, most of the electrons delivered to the hydrogenase originate from water oxidation by PSII, (ii) a faster onset of anaerobiosis preserves PSII from irreversible photoinhibition, and (iii) mutants with enhanced respiratory activity should be considered for better photobiological H2 production.
Solar fuels are an attractive concept for development of future renewable energy systems. Among other fuels, H2 is considered to be one of the most effective and clean fuels (1–3). Solar-driven H2 production by photosynthetic microorganisms (photobio-H2 production) is a viable alternative that complements the proposed chemical technologies. Green algae and cyanobacteria can, using water as an electron source via photosynthesis, produce H2 with specific H2-evolving enzymes (hydrogenases) coupled to the photosynthetic machinery (4–6).
Although green algae possess a very active hydrogenase enzyme compared with other organisms (the turnover rate of the algal Fe-Fe hydrogenase is in thousands per second, 100-fold higher than that of other hydrogenases), direct light-to-H2 conversion efficiency is very low (7, 8). Thus, this is not a main metabolic process. Moreover, H2 formation requires anaerobic conditions in the cell because the hydrogenase activity is sensitive to the presence of O2. The consequence is that oxygenic photosynthesis cannot easily be directly coupled to H2 evolution in green algae.
Melis and coworkers reported a two-stage process based on sulfur (S) deprivation in Chlamydomonas reinhardtii, which allowed the separation of the photosynthetic reactions from H2 formation (9). Cell cultivation in sealed conditions in media deprived of sulfur allowed light-dependent H2 evolution for several days. The efficiency was the highest for photobiological systems reported so far (2%) (8). Under these conditions photosynthesis is down-regulated and O2 consumption overtakes O2 evolution, creating anaerobic conditions in the cell. This in turn activates hydrogenase expression and activity (10, 11).
Cells of C. reinhardtii undergo morphological changes and accumulate starch under S deprivation (12). Both the light and the dark reactions of photosynthesis are down-regulated (9, 12, 13). The amount of Rubisco is drastically reduced during the first 24 h, leading to cessation of the CO2 fixation (12, 14). Photosynthetic electron transfer reactions are also inhibited (9, 15, 16). This is mostly associated with changes in the photosystem II (PSII) complex that are not well characterized. This is unfortunate because a fraction of the electrons delivered to the hydrogenase have been suggested to originate from water oxidation in PSII (17).
PSII is a large multisubunit complex that drives photooxidation of water and reduction of the plastoquinone (PQ) pool, thus initiating the electron transport chain in the thylakoid membrane (18). Apart from light capture and charge separation, PSII possesses a defined sequence of electron and proton transfer reactions (19). In addition, the PSII complex undergoes complex regulation in response to environmental stress factors, especially high light intensities (20, 21). In this study we analyze the changes in the PSII content and activity under H2 production conditions in the wild-type (WT) and the Stm6 mutant of C. reinhardtii. The latter was earlier reported to exhibit enhanced H2 yield.* We report and quantify the decrease in the amount of PSII and describe a regulation of the acceptor side reactions in PSII. The latter is controlled by changes in the redox state of the quinone acceptors in PSII and of the PQ pool occurring during S deprivation and subsequent H2 production.
Results
O2 and H2 Balance During S Deprivation of WT and Stm6 Mutant Cells.
Under photoheterotrophic conditions, the growth rates of the WT and the Stm6 mutant strains were not different. After the cells were transferred to the S-deprived conditions, the cell number decreased by less than 10% by the end of the experiment. The total amount of chlorophyll (Chl) also decreased by ∼20%, and the Chl a/b ratio increased from 2.60 to 3.06 for the WT and from 2.30 to 2.94 for the Stm6 mutant. This is explained by partial degradation of the LHCII antenna (12).
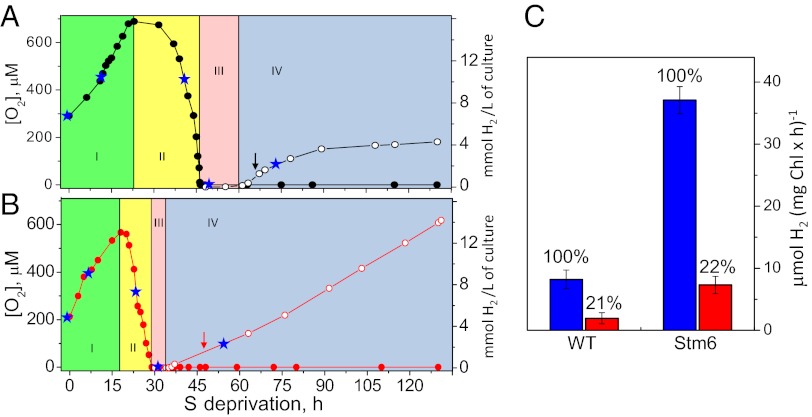
In both the WT and the Stm6 mutant cells, the rate of O2 evolution significantly decreased and the rate of respiration increased in the beginning of S deprivation, leading to establishment of anaerobic conditions in the culture. Interestingly, due to the lower initial rate of O2 evolution and the higher initial rate of respiration in the Stm6 mutant, anaerobiosis was achieved much faster in the Stm6 mutant cells than in the WT cells. Fig. 1 A and B shows the concentration of dissolved O2 in the medium during S deprivation in the sealed flasks. First, the concentration of O2 in the medium increased due to still photosynthetically active cells during this period. After this, the concentration of O2 started to decrease, finally reaching anaerobicity. The decrease in the O2 concentration reflects mainly a decrease in the O2 evolution capacity from PSII and partly the increased respiration (by 25–30%) in both WT and Stm6 mutant cells. The concentration of O2 remained at zero during the rest of the experiment (up to 130 h, Fig. 1 A and B). A few hours after the anaerobic conditions were established, H2 evolution was observed in both strains.
Fig. 1.
Changes in the concentration of dissolved O2 (filled circles) and produced H2 (open circles) during incubation of WT (A, black circles) and the Stm6 mutant (B, red circles) of C. reinhardtii under S-deprived conditions in sealed flasks. Reflecting the O2 and H2 concentrations, the time course was divided into four phases: I—the O2 evolution phase; II—the O2 consumption phase; III—the anaerobic phase; and IV—the H2 production phase. The blue stars in each phase indicate the time points for EPR and fluorescence experiments. The sample at t = 0 was taken as a control. (C) The effect of the addition of 20 μM DCMU on the rate of H2 production. The aliquots of the cell culture were taken as described in the text at the time points indicated by arrows in A and B. The amount of H2 formed in the respective strains in the absence of DCMU (blue) is set as 100%. The fraction of H2 formed in the presence of DCMU (red) was similar in both WT and the Stm6 mutant whereas the net amount of H2 formed was four times greater in the mutant.
Our observed changes in the O2 and H2 concentrations during cultivation of C. reinhardtii cells in S-deprived conditions are similar to those observed in an earlier study (24) where the process was dissected in four different phases: the O2 evolution phase (I), the O2 consumption phase (II), the anaerobic phase (III), and the H2 production phase (IV) (Fig. 1 A and B) (24). All four phases were observed in the WT and the Stm6 mutant. However, the time dependence of the phases was different. First, the anaerobic phase was established in much shorter time for the Stm6 mutant (by 15 h if compared with the WT cells, Fig. 1 A and B). This reflects a combined effect of the decreased O2 evolution capacity and the increased respiration rate in the mutant. Second, phase III was much shorter in the Stm6 mutant cells; the H2 production was detected only a few hours after the onset of anaerobiosis. Third, H2 production was also much more effective in the Stm6 mutant than in the WT [as shown before (23)] and continued with an increasing rate during the entire 130 h time course of the experiment (Fig. 1B).
3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) is a specific inhibitor of PSII that disrupts the electron transfer from QA− to QB by occupying the QB site. It has been reported that the H2 production during S-deprived cultivation of C. reinhardtii cells is partially inhibited by the addition of DCMU (12, 17, 23, 25–27), which indicates that electrons from PSII are used by hydrogenase (12, 17, 27). This link between photosynthetic water oxidation and hydrogen evolution, and the mechanism behind it, is very important for the understanding of photobiological hydrogen production and its further development.
We have tested if this DCMU effect occurs also under our conditions and the results are shown in Fig. 1C. The samples were withdrawn from the culture at the time points indicated by arrows in Fig. 1A (WT) and Fig. 1B (Stm6 mutant) and incubated in the presence or absence of DCMU. In both the WT and the Stm6 mutant, ∼80% of the rate of H2 evolution was lost upon the addition of DCMU (Fig. 1C). Thus, our results indicate a significant involvement of PSII in photosynthetic hydrogen evolution.
The molecular mechanism of how this occurs has been addressed here with selective spectroscopic analysis. Measuring points to assess PSII reactions were selected during the four different phases (indicated by stars in Fig. 1 A and B), and at each time point samples were anaerobically withdrawn from the main culture for further analysis.
The control point was at the beginning of the S deprivation (t = 0). Here the properties of PSII were essentially the same as in samples analyzed just before the S deprivation procedure was commenced. In phase III, the samples were taken out within 10 min after the anaerobic conditions were established but clearly before we observed any production of H2 (11).
Electron Paramagnetic Resonance Measurements.
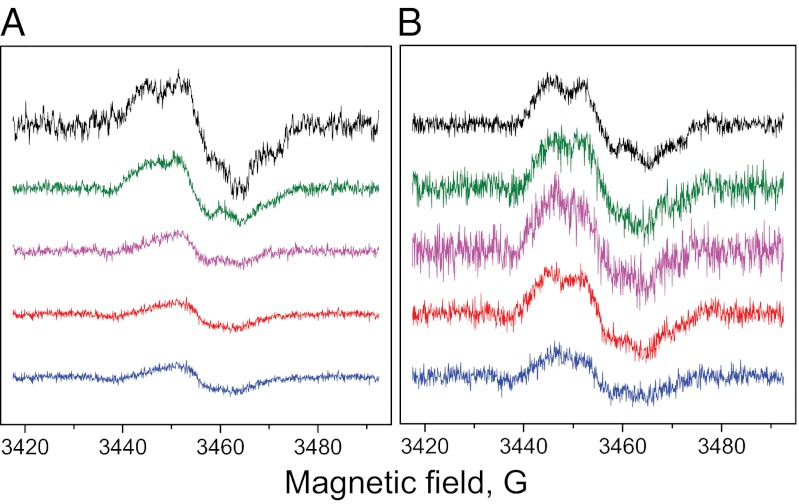
The amount of PSII in cells can be directly quantified by electron paramagnetic resonance (EPR) spectroscopy (28). This is done on the basis of the tyrosine D• radical, which amounts to one radical spin per PSII reaction center. The results are shown in Fig. 2 and Table 1. The amount of PSII in the control samples was set as 100%. In the WT cells it decreased to 54% in the O2-evolving phase (phase I) and to 25% in the O2-consumption phase. Interestingly, the concentration of PSII remained at this level during the anaerobic (III) and H2 production (IV) phases (Fig. 2 and Table 1). Thus, the number of PSII centers in the WT cells decreased by ∼75% during S deprivation.
Fig. 2.
EPR spectra of tyrosine D• taken from intact cells of the WT (A) and Stm6 mutant (B) of C. reinhardtii at different stages of S deprivation: control (time = 0, black); the O2-evolving phase (I, green); the O2 consumption phase (II, magenta); the anaerobic phase (III, red); and the H2 production phase (IV, blue). The spectra were normalized to the same Chl concentration. The full induction of the signal was achieved as described in ref. 28. EPR conditions were: microwave frequency 9.76 GHz, microwave power 8 mW, modulation amplitude of 5 G, and room temperature.
Table 1.
Changes in the PSII amount during different phases of S deprivation in WT and Stm6 mutant cells of C. reinhardtii
| Tyrosine D• (%)* |
||
| Phase in S deprivation | WT | Stm6 |
| Control | 100 | 100 |
| O2-evolving phase (I) | 54 | 102 |
| O2 consumption phase (II) | 25 | 102 |
| Anaerobic phase (III) | 22 | 98 |
| H2 production phase (IV) | 23 | 52 |
PSII content was estimated from measurements of tyrosine D•.
Data are normalized on the basis of the Chl content in the respective sample as described in ref. 28 (Material and Methods).
The situation was different in the Stm6 mutant. The amount of PSII did not change during first three phases of the S deprivation process (Fig. 2 and Table 1). Moreover, the amount of PSII decreased to only 52% during the H2 production phase in the Stm6 mutant (Fig. 2 and Table 1). Thus, the amount of PSII was much less affected in the Stm6 mutant than in the WT during the S deprivation process. The different remaining PSII content (23% in the WT; 52% in the Stm6 mutant) is clearly very important when the cells have reached the capacity to produce H2 (phase IV).
Flash-Induced Fluorescence Decay Measurements.
The functional capacity of the PSII centers during the different phases of S deprivation was analyzed by flash-induced fluorescence decay kinetic measurements in WT and Stm6 mutant cells. This method reports on the electron transport properties of PSII and can be used to monitor both the acceptor- and the donor-side reactions (29, 30). Immediately after the flash, the fluorescence rises from the F0 level to the Fmax level (Fig. 3). This reflects the charge separation in PSII and the stabilization of the electron on the first quinone acceptor, QA. This results in the formation of a high-fluorescence state. The consequent decay of variable fluorescence demonstrates how the electron leaves QA− in the particular sample (29, 30).
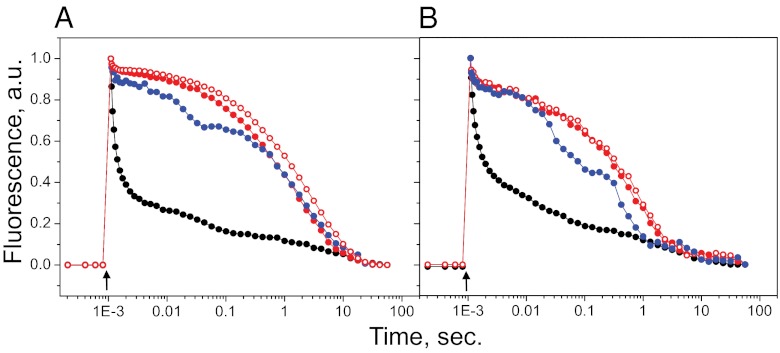
Fig. 3.
Normalized flash-induced fluorescence decay traces from WT (A) and the Stm6 mutant (B) of C. reinhardtii cells in control samples (I, black circles); samples from the anaerobic phase (III, closed red circles); samples from the anaerobic phase in the presence of 20 μM DCMU (III, open red circles); and samples from the H2 production phase (IV, blue circles). The arrows indicate the time when the flash was applied.
In the control sample from the WT cells, more than 80% of the fluorescence decayed in the microsecond to millisecond time range (Fig. 3A, black circles). This fast decay of the variable fluorescence is normal and reflects fast and efficient forward electron transfer from QA− to QB (resulting in QB−) in the majority of the PSII centers. In the oxygen evolution (phase I) and consumption (phase II) phases, this forward electron transfer became sequentially slower. When the cells reached the anaerobic phase (III), the fluorescence decay kinetics were dramatically different (Fig. 3A, closed red circles). Instead of the fast decay indicative of forward electron transfer, the decay kinetics were dominated by slow reactions occurring in the hundreds of milliseconds to seconds time range. Such slow decay kinetics were typical for recombination reactions between QA− and the S2 state of the water-oxidizing complex in PSII (29–31). Indeed, very similar kinetics were observed when the flash-induced fluorescence decay was measured in the same sample but in the presence of DCMU, which blocks forward electron transfer between QA− and QB (Fig. 3A, open red circles). The fluorescence decay kinetics in the presence of DCMU are well understood and reflect recombination between QA− and the S2 state (29–31). Thus, from the almost identical kinetics we conclude that the forward electron transfer from QA− was completely blocked in the anaerobic phase (III) during S deprivation of the WT cells. This indicates that the PQ pool is completely reduced in the anaerobic phase. Furthermore, from the observation of the flash-induced fluorescence we can also conclude that the 22–23% of PSII centers that actually remained (Table 1) were active both in reducing QA and in forming the S2 state.
When the cells reached the H2 production phase (IV), the kinetics of the flash-induced fluorescence changed again. In 35–40% of the remaining PSII centers, new decay kinetics occurring in the few millisecond time scale appeared (Fig. 3A, blue circles). These kinetics are indicative of rather slow, but existing, forward electron transfer from QA− in PSII. We propose that these kinetics reflect rebinding of QB to its site and subsequent electron transfer from QA− to the newly bound QB (see refs. 29–32). The consequence of this assignment is that a significant fraction of the PQ pool somehow must have become oxidized in the H2 production phase.
The situation in the Stm6 mutant cells was similar during the first three phases of S deprivation. The control sample showed fast decay of the variable fluorescence, reflecting active PSII with effective forward electron transfer from QA− (Fig. 3B, black circles). The kinetics were similar to those in the WT. In the oxygen evolution (I) and consumption (II) phases, this forward electron transfer was still dominant but became sequentially slower. In the anaerobic phase (III), the fluorescence decay kinetics were again dominated by the slow decay (Fig. 3B, closed red circles) that here also was similar to the decay in the presence of DCMU (Fig. 3B, open red circles). The slow kinetics were indicative of recombination reactions between QA− and the donor side of PSII and reflects blocked forward electron transfer from QA− to QB (29–32).
In the H2 production phase (IV) in the Stm6 mutant cells, the opening of the forward electron transfer from QA− was again observed. The forward electron transfer involved a higher fraction of the PSII centers in the Stm6 mutant than in the WT cells and about 60% of the remaining PSII centers showed millisecond fluorescence decay kinetics in the H2 production phase (Fig. 3B, blue circles).
Discussion
The aim of this work was to study how light-driven photosynthesis is regulated in response to H2 production under S deprivation in the green algae C. reinhardtii. Despite the fact that this process requires separation of photosynthesis and H2 evolution into different time stages, it remains the most effective known photobiological way of converting solar energy into H2 (4, 7, 8, 33). The separation of O2 evolution (photosynthesis) and H2 evolution, however, is not complete, and partial photosynthetic light reactions still take place under S-deprived conditions as was observed earlier (9, 12, 15, 16, 24, 27). This is of potential importance because, in the ideal photobiological system, electrons and protons for H2 production must originate from the water-splitting reaction, thus leaving PSII as the focus of this study.
PSII and H2 Evolution in WT cells.
It is known that various photosynthetic reactions are down-regulated to a different extent during the S deprivation procedure. The level of Rubisco became undetectable after 40 h of S deprivation resulting in inhibition of the CO2 fixation (12, 34). PSII is the most affected complex involved in the light reactions as was shown by the decrease in O2 evolution, fluorescence parameters, and the amount of the D1 protein (9, 12, 15, 16, 24). PSI, the cytochrome b6f complex, and different antenna proteins are less affected (9, 12).
Decrease in O2 evolution and increased respiration rates lead to establishment of anaerobic conditions in cells. This is illustrated in Fig. 1A where the concentration of dissolved O2 in the medium first increased due to O2 evolution (O2-evolving phase I) and then decreased when the diminishing O2 evolution by PSII was overcome by respiration (O2 consumption phase II). A short time after anaerobiosis was established (III), the light-driven H2 production commenced (Fig. 1A, H2 production phase IV). This reflects the expression of the hydrogenase that has been shown to occur within 20 min after the onset of anaerobiosis under conditions similar to ours (11).
It is clear that the metabolic changes that lead to H2 evolution are very dependent on the O2 balance in the cell. Therefore, it is important to follow changes in the PSII properties during the S deprivation procedure. We have used EPR spectroscopy to quantify PSII centers in our samples. The advantage of this method is that it can be applied in vivo on intact cells and that it can be used quantitatively with high precision (28).
Our measurements show that the amount of PSII in the WT cells had already decreased at the beginning of S deprivation (Table 1). The amount of PSII decreased to 54% during the O2 evolution phase and to 25% during the O2 consumption phase and stayed at a similar level during consequent phases. Thus, the amount of PSII was decreased by ∼75% during the first two phases of the S deprivation procedure (Table 1).
The reason for this dramatic decrease in PSII amount during the first 45 h is most probably the distorted balance between photoinhibition and repair of PSII. Our illumination intensity of 80 μE⋅m−2⋅s−1 was chosen for maximal H2 yield at our conditions. Photoinhibition is a steady-state process that takes place even at these moderate light intensities. It results in the inhibition of the electron transport reactions in PSII, disassembly of the complex, and O2-dependent degradation of PSII proteins, most notably, the D1 protein (20, 21, 35, 36). Repair of the photoinhibited PSII centers requires the de novo protein synthesis. It can probably still take place during the first few hours of the O2-evolving phase but will thereafter be completely halted due to the lack of sulfur and inability to synthesize the essential amino acids (12). This eventually results in a substantial (75%) decrease in the amount of PSII during the O2 evolution and consumption phases (Fig. 2 and Table 1).
Surprisingly, in the anaerobic (III) and H2 production (IV) phases PSII remains at ∼25% of the original level (Table 1). The reason for this is probably the anaerobic environment. It is known that D1 protein degradation in normal light is mainly O2-dependent, reflecting interaction between Chl triplets and O2, leading to damaging singlet oxygen formation (35, 36). It is also known that, in the absence of O2, photoinhibition results in an inhibited form of PSII with no bound QB and semistable QA− (37, 38) whereas the D1 protein seems unharmed. Such photoinhibition is reversible, and these PSII centers are still able to restore their electron transport capacity, providing that, first, the PQ pool and, then, QA− are somehow reoxidized (37, 38).
The functional state of the remaining 25% of PSII centers needs to be analyzed further, especially with respect to H2 production in the WT C. reinhardtii cells. Our experiments with DCMU, which binds to the QB site in PSII and blocks forward electron transfer, clearly show that ∼80% of the H2 formation was inhibited in the presence of DCMU (Fig. 1C). This effect was known before, and reported values for the DCMU inhibition vary from 20 to 85%, probably reflecting varying experimental conditions (12, 23, 25–27). However, the question of whether the electrons delivered to the hydrogenase during the light-induced H2 evolution really originate from PSII activity has not been fully addressed so far.
Our fluorescence data clarify this question. The strength of the flash-induced fluorescence decay measurements is that it allows monitoring of the electron transfer specifically from QA− after a single flash. These measurements, shown in Fig. 3A, indicate that the 25% of the PSII centers that are left in the anaerobic phase are not able to reduce QB. They are behaving similarly to DCMU-treated centers; i.e., they are not competent in the forward electron transfer from QA− (Fig. 3A). These centers behave similarly to the reversibly photoinhibited PSII centers (under anaerobic conditions), which we described briefly above. They are fully competent in charge separation to QA−. Moreover, the decay kinetics are indicative of a functional CaMn4 cluster capable of normal S-state advancement when the acceptor side is reactivated again (29–32). The reason why the electron transfer in these remaining PSII centers is blocked beyond QA is therefore not because there are modifications within PSII. Instead, it reflects changes in the redox state of the PQ pool, and the fluorescence measurements suggest that the PQ pool is fully reduced in the anaerobic state (III).
It is known that S deprivation changes the redox state in the cells. The redox potential in the TAP-S medium drops from 400 to –100 mV during the anaerobic phase (III) (15, 24). This is the result of the changed metabolism in the S-deprived cells, the decreased activity of Rubisco, accumulation of starch, and contribution of electrons from the starch catabolism to the electron transport chain in the thylakoid membrane. These cumulative changes lead to a very reduced state of the PQ pool. The lack of oxidized PQ results in that the QB site in PSII is most probably empty. The result is the complete block of the forward electron transfer from QA−, which we observe in the flash-induced fluorescence measurements.
However, this situation is changed with the expression of hydrogenase, which starts shortly after the onset of anaerobiosis (11). Our observations indicate that the PQ pool slowly becomes slightly oxidized. This results in the opportunity for oxidized PQ to rebind to PSII, which, in turn, allows the forward electron transfer from QA− as observed by the appearance of the fast decay phase in the fluorescence kinetics in samples from the H2 production phase (Fig. 3A, blue). This fast decay phase cannot be attributed to normal QA− to QB electron transfer. Instead, it is more compatible with the kinetics of electron transfer from QA− to QB, which first has to bind to PSII (29, 32). This is not surprising because the redox state of the PQ pool is still far from normal under these H2 production conditions. We propose that the electron pressure on the electron transport chain is partially relieved in H2 evolution by hydrogenase-drawing electrons from the PQ pool that have become completely reduced during the earlier phases (I–III, Fig. 1).
This is the first direct experimental evidence that PSII provides electrons to hydrogenase during light-induced H2 evolution in the S-deprived system. Our estimation from the amplitude of this fast phase (Fig. 3A, blue) is that only ∼9% of the PSII centers that were present from the start of the experiment contribute to H2 production during S deprivation in WT C. reinhardtii cells at the time point at which the measurements were conducted (Fig. 1A, star in phase IV). The other PSII centers are either degraded during phases I and II (77%, Table 1) or still not active in forward electron transfer from QA− (14%).
PSII and H2 Evolution in Stm6 Cells.
The situation was similar but also different in the Stm6 mutant. This mutant is reported to have a modified respiratory metabolism, elevated starch reserves, and low O2 evolution. In addition, it was found to be defective in the so-called “state transition” and thus lacking ability for cyclic electron flow around PSI. The mutant was also reported to have more than five times the elevated H2 production rates than the WT cells (23).
We obtained similar results with the Stm6 mutant. The anaerobic condition was attained much faster in the mutant than in the WT. This reflects a combined effect of the lower initial O2 evolution rate and the higher respiration rate. After only 30 h, anaerobiosis was created in the sealed cultures (Fig. 1B). Importantly, the H2 formation had already started after less than 5 h of anaerobiosis.
In our experiments, the H2 evolution was about four times higher in the Stm6 mutant when compared with the WT (Fig. 1). We correlate this higher H2 evolution yield to the higher remaining PSII content in the Stm6 mutant. During the H2 production phase (IV), PSII amounted to 52% of its original level (Table 1). Thus, there are more PSII centers left that potentially are able to provide electrons for the H2 production in the Stm6 mutant cells (Table 1). It is interesting to discuss why so much PSII is left in the mutant compared with the WT despite the identical experimental conditions. We propose that this reflects the altered balance between decreased O2 evolution and respiration. This results in a much faster onset of anaerobiosis under which PSII is not irreversibly damaged. Less PSII is consequently damaged during phases I and II, which are much shorter in the mutant. Under anaerobiosis (III), PSII is no longer irreversibly damaged, and, when the electron pressure on the PQ pool is relieved, more PSII centers are able to perform forward electron transport, eventually all of the way to hydrogenase.
Despite the higher rates of H2 evolution and the higher PSII content, ∼80% of H2 evolution was inhibited by the DCMU addition (Fig. 1C) similar to in the WT. This leads again to the question, what is the functional status of the PSII centers that remain in the H2 production phase of the Stm6 mutant? Our fluorescence measurements showed that PSII was “closed” in the mutant during the anaerobic phase, effectively eliminating the electron supply from QA− to QB and the PQ pool. However, following the onset of H2 formation, the opening of the forward electron transport on the acceptor side in PSII was much more effective in the mutant than in WT: as much as 60% of the fluorescence decay became faster and can thus be attributed to the electron flow from QA− to QB (Fig. 3B). That leaves us with conclusion that ∼32% of the original PSII centers (60% of the remaining 52% of PSII) contribute to H2 production during the S deprivation procedure in Stm6 cells. This is about four times more than in the WT (9%) and is consistent with the difference that we have observed in the H2 evolution rates (four times, Fig. 1).
Conclusions.
We have analyzed the activity of PSII during S deprivation in the WT and the Stm6 mutant of C. reinhardtii. In the H2 production phase in particular, we quantified and analyzed the function of the remaining, reactivated PSII centers. They are competent in charge separation and S-state advancement and are able to provide electrons to the PQ pool. Our estimation of the activity of these remaining PSII centers indicates that the water splitting by PSII supplies the majority of electrons for H2 synthesis. This estimation is corroborated by our experiments in which H2 production was inhibited by the addition of DCMU. Our results also indicate that the hydrogenase uses electrons from the PQ pool during the H2 production phase. This partly removes the block in PSII electron transport from QA− to plastoquinone, thereby permitting electron flow from water oxidation in PSII to hydrogen.
Moreover, the rate of H2 production is proportional to the amount of operational PSII present. In the Stm6 mutant where the amount of PSII was four times higher than in the WT during the H2 production phase, the amount of H2 produced was correspondingly four times higher. The higher amount of the remaining PSII centers reflects the modified redox environment in the thylakoid membrane of the Stm6 mutant (23), which leads to an earlier onset of anaerobiosis. The earlier anaerobiosis is an important prerequisite for preservation of PSII during illumination under S-deprived conditions.
Based on our spectroscopic analysis we can also explain how enhanced respiration rates lead to higher rates of H2 production. Enhanced respiration induces a faster onset of anaerobiosis. The absence of oxygen switches off irreversible photoinhibition and D1-protein degradation, which is oxygen dependent (38). This leaves more PSII centers. Under anaerobic conditions these remaining PSII centers exist in the “reversibly inhibited state” that was described in ref. 38. They are competent in all electron transport reactions except the reduction of the PQ pool. Thereby, an earlier anaerobiosis creates conditions that protect PSII centers from irreversible photoinhibition due to D1-protein damage and degradation. When expression and function of the hydrogenase relieve the electron pressure on the PQ pool, these reversibly inhibited PSII centers are reactivated. Therefore, earlier onset of anaerobiosis, as for example in the Stm6 mutant, will preserve more PSII centers capable of water oxidation after reactivation.
This observation is important because it is opens up a way to improve the H2 production in green algae, which is based on enhanced respiratory reactions, similar to Stm6 and some other mutant systems (23, 27, 39). The search for existing mutations or engineering of new ones, which can create earlier and extended anaerobic conditions, will potentially overcome two major problems. First, it will reduce the damaging effect of oxygen on the hydrogenase. Second, the absence of oxygen will protect the D1 protein in PSII against damage. Thereby, the electron source, PSII, is preserved on an elevated level, allowing more efficient photobiological H2 production.
Materials and Methods
Culture Growth and S Deprivation.
WT and the Stm6 mutant of C. reinhardtii were grown photoheterotrophically in TAP medium in a shaking incubator at 25 °C under cool white fluorescence light of 80 μE⋅m−2⋅s−1 intensity. Cells were collected in the middle of the logarithmic growth phase, washed three times in TAP-S medium (9, 26), and suspended in 300-mL sealed conical flasks (bio-reactors) (25 °C) at the density of ∼6–7 × 106 cells/mL. For H2 evolution experiments, the culture in the TAP-S medium was incubated under light of 80 μE⋅m−2⋅s−1 intensity and with constant stirring in the sealed bioreactors for the indicated period and up to 130 h.
Measurements of Dissolved Oxygen, Respiration, Oxygen, and Hydrogen Production.
The concentration of dissolved oxygen in the liquid culture was measured directly with an oxygen analyzer (MAPK-302T). The rates of respiration and oxygen evolution in C. reinhardtii cells were measured with a Clark electrode at 25 °C. Aliquots of 1 mL were taken from the bioreactor and placed in the Clark electrode cell. After 2 min of adaptation, the rate of the respiration was recorded in the dark for 3 min. Oxygen evolution was measured after the addition of 5 mM NaHCO3 and subsequent 2-min dark incubation with saturating white light for 5 min.
The amount of H2 in the gas phase was determined with a Clarus 500 gas chromatograph complete with a Molecular Sieve 5A 60/80 Mesh column (Perkin-Elmer) and Ar as a carrier gas. The effect of DCMU on the H2 production was measured 20 h after anaerobic conditions had been established. At the position indicated by arrows in Fig. 1 A and B, samples were anaerobically withdrawn, and 4 mL of the cell culture was transferred into 7-mL gas-tight Ar-flushed glass vials. After 3 h incubation under illumination and constant stirring at 25 °C, 20 μM DCMU was added (this concentration is sufficient to inhibit all PSII centers at the cell density used in our experiments). One hour after the DCMU addition, the amount of produced H2 in the gas phase was determined.
Variable Fluorescence Decay Kinetics.
Flash-induced variable fluorescence decay kinetics was measured with a FL3000 double modulated fluorometer (Photon Systems Instruments) as described in ref. 31. One mL of the cell suspension (∼15 μg of Chl/mL) was taken from the culture at the time points indicated by stars in Fig. 1 and dark-adapted for 5 min before the actinic flash was applied.
EPR Spectroscopy.
EPR measurements were performed in a flat cell with a Bruker ELEXYS E500 spectrometer equipped with a SuperX EPR049 microwave bridge and a SHQ4122 cavity as described in ref. 28. At each time point indicated by a star in Fig. 1, the entire cell culture from one bioreactor was concentrated to ∼200 μg of Chl/mL by centrifugation at 1,620 × g and frozen at –80 °C before the use.
For all measurements, in the first two time points indicated by stars in Fig. 1, samples were removed from the bioreactor aerobically. In the following time points in Fig. 1, all procedures were done under anaerobic conditions.
Acknowledgments
We thank Dr. Olaf Kruse for providing us with the Stm6 mutant strain and Dr. Ian Ross for useful discussions. We thank the Swedish Research Council, the Swedish Energy Agency, the Knut and Alice Wallenberg Foundation, and the AquaFEED project from Nordic Energy Research for financial support. A.V. further acknowledges the Swedish Institute for a research stipend.
Footnotes
The authors declare no conflict of interest.
*The Stm6 mutant (state transition mutant 6) with modified light harvesting antenna properties and lacking cyclic electron flow was first isolated and described in (22). The mutant is lacking the Moc1 gene involved in the assembly of the mitochondrial respiratory chain in the light (22) and has been shown to have extended H2 production under S deprivation (23).
This article is a PNAS Direct Submission.
References
- 1.Hankamer B, et al. Photosynthetic biomass and H2 production by green algae: From bioengineering to bioreactor scale-up. Physiol Plant. 2007;131(1):10–21. doi: 10.1111/j.1399-3054.2007.00924.x. [DOI] [PubMed] [Google Scholar]
- 2.Mathews J, Wang G. Metabolic pathway engineering for enhanced biohydrogen production. Int J Hydrogen Energy. 2009;34(17):7404–7416. [Google Scholar]
- 3.Magnuson A, et al. Biomimetic and microbial approaches to solar fuel generation. Acc Chem Res. 2009;42(12):1899–1909. doi: 10.1021/ar900127h. [DOI] [PubMed] [Google Scholar]
- 4.Ghirardi ML, Dubini A, Yu J, Maness P-C. Photobiological hydrogen-producing systems. Chem Soc Rev. 2009;38(1):52–61. doi: 10.1039/b718939g. [DOI] [PubMed] [Google Scholar]
- 5.Hemschemeier A, Happe T. Alternative photosynthetic electron transport pathways during anaerobiosis in the green alga Chlamydomonas reinhardtii. Biochim Biophys Acta. 2011;1807(8):919–926. doi: 10.1016/j.bbabio.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Horner DS, Heil B, Happe T, Embley TM. Iron hydrogenases: Ancient enzymes in modern eukaryotes. Trends Biochem Sci. 2002;27(3):148–153. doi: 10.1016/s0968-0004(01)02053-9. [DOI] [PubMed] [Google Scholar]
- 7.Esper B, Badura A, Rögner M. Photosynthesis as a power supply for (bio-)hydrogen production. Trends Plant Sci. 2006;11(11):543–549. doi: 10.1016/j.tplants.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Rupprecht J, et al. Perspectives and advances of biological H2 production in microorganisms. Appl Microbiol Biotechnol. 2006;72(3):442–449. doi: 10.1007/s00253-006-0528-x. [DOI] [PubMed] [Google Scholar]
- 9.Melis A, Zhang L, Forestier M, Ghirardi ML, Seibert M. Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol. 2000;122(1):127–136. doi: 10.1104/pp.122.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghirardi ML, Togasaki RK, Seibert M. Oxygen sensitivity of algal H2- production. Appl Biochem Biotechnol. 1997;63–65:141–151. doi: 10.1007/BF02920420. [DOI] [PubMed] [Google Scholar]
- 11.Happe T, Kaminski A. Differential regulation of the Fe-hydrogenase during anaerobic adaptation in the green alga Chlamydomonas reinhardtii. Eur J Biochem. 2002;269(3):1022–1032. doi: 10.1046/j.0014-2956.2001.02743.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Happe T, Melis A. Biochemical and morphological characterization of sulfur-deprived and H2-producing Chlamydomonas reinhardtii (green alga) Planta. 2002;214(4):552–561. doi: 10.1007/s004250100660. [DOI] [PubMed] [Google Scholar]
- 13.Hemschemeier A, Melis A, Happe T. Analytical approaches to photobiological hydrogen production in unicellular green algae. Photosynth Res. 2009;102(2–3):523–540. doi: 10.1007/s11120-009-9415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemschemeier A, Fouchard S, Cournac L, Peltier G, Happe T. Hydrogen production by Chlamydomonas reinhardtii: An elaborate interplay of electron sources and sinks. Planta. 2008;227(2):397–407. doi: 10.1007/s00425-007-0626-8. [DOI] [PubMed] [Google Scholar]
- 15.Antal TK, et al. The dependence of algal H2 production on Photosystem II and O2 consumption activities in sulfur-deprived Chlamydomonas reinhardtii cells. Biochim Biophys Acta. 2003;1607(2–3):153–160. doi: 10.1016/j.bbabio.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Volgusheva AA, et al. Examination of chlorophyll fluorescence decay kinetics in sulfur deprived algae Chlamydomonas reinhardtii. Biochim Biophys Acta. 2007;1767(6):559–564. doi: 10.1016/j.bbabio.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 17. Ghirardi ML, Kosourov S, Tsygankov A, Seibert M (2000) Two-phase photobiological algal H2-production system. Proceedings of the 2000 DOE Hydrogen Program Review (San Ramon, CA), pp 1–13. NREL/CP-570–28890. Available at www.energy.gov/hydrogenandfuelcells/pdfs/28890f.pdf.
- 18.Wydrzynski TJ, Satoh K, editors. Photosystem II: The Light-Driven Water: Plastoquinone oxidoreductase. Berlin: Springer; 2005. [Google Scholar]
- 19.Rappaport F, Diner BA. Primary photochemistry and bioenergetics leading to oxidation of the (Mn)4Ca cluster and to evolution of molecular oxygen in photosystem II. Coord Chem Rev. 2008;252:259–272. [Google Scholar]
- 20.Vass I, Aro E-M. Photoinhibition of photosynthetic electron transport. In: Renger G, editor. Primary Processes in Photosynthesis: Basic Principles and Apparatus. Cambridge, UK: Royal Society of Chemistry; 2008. pp. 393–425. [Google Scholar]
- 21.Mamedov F, Styring S. Logistics in the life cycle of photosystem II during maturation: Lateral movement in the thylakoid membrane and activation of the electron transfer. Physiol Plant. 2003;119:328–336. [Google Scholar]
- 22.Schönfeld C, et al. The nucleus-encoded protein MOC1 is essential for mitochondrial light acclimation in Chlamydomonas reinhardtii. J Biol Chem. 2004;279(48):50366–50374. doi: 10.1074/jbc.M408477200. [DOI] [PubMed] [Google Scholar]
- 23.Kruse O, et al. Improved photobiological H2 production in engineered green algal cells. J Biol Chem. 2005;280(40):34170–34177. doi: 10.1074/jbc.M503840200. [DOI] [PubMed] [Google Scholar]
- 24.Kosourov S, Tsygankov A, Seibert M, Ghirardi ML. Sustained hydrogen photoproduction by Chlamydomonas reinhardtii: Effects of culture parameters. Biotechnol Bioeng. 2002;78(7):731–740. doi: 10.1002/bit.10254. [DOI] [PubMed] [Google Scholar]
- 25.Kosourov S, Seibert M, Ghirardi ML. Effects of extracellular pH on the metabolic pathways in sulfur-deprived, H2-producing Chlamydomonas reinhardtii cultures. Plant Cell Physiol. 2003;44(2):146–155. doi: 10.1093/pcp/pcg020. [DOI] [PubMed] [Google Scholar]
- 26.Antal TK, Volgusheva AA, Kukarskih GP, Krendeleva TE, Rubin AB. Relationships between H2 photoproduction and different electron transport pathways in sulfur-deprived Chlamydomonas reinhardtii. Hydrogen Energy. 2009;34:9087–9094. [Google Scholar]
- 27.Scoma A, et al. Sustained H₂ production in a Chlamydomonas reinhardtii D1 protein mutant. J Biotechnol. 2012;157(4):613–619. doi: 10.1016/j.jbiotec.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 28.Danielsson R, Albertsson P-Å, Mamedov F, Styring S. Quantification of photosystem I and II in different parts of the thylakoid membrane from spinach. Biochim Biophys Acta. 2004;1608(1):53–61. doi: 10.1016/j.bbabio.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Vass I, Kirilovsky D, Etienne A-L. UV-B radiation-induced donor- and acceptor-side modifications of photosystem II in the cyanobacterium Synechocystis sp. PCC 6803. Biochemistry. 1999;38(39):12786–12794. doi: 10.1021/bi991094w. [DOI] [PubMed] [Google Scholar]
- 30.Mamedov F, Stefansson H, Albertsson P-A, Styring S. Photosystem II in different parts of the thylakoid membrane: A functional comparison between different domains. Biochemistry. 2000;39(34):10478–10486. doi: 10.1021/bi992877k. [DOI] [PubMed] [Google Scholar]
- 31.Mamedov F, Nowaczyk MM, Thapper A, Rögner M, Styring S. Functional characterization of monomeric photosystem II core preparations from Thermosynechococcus elongatus with or without the Psb27 protein. Biochemistry. 2007;46(18):5542–5551. doi: 10.1021/bi7000399. [DOI] [PubMed] [Google Scholar]
- 32.Rova M, Mamedov F, Magnuson A, Fredriksson P-O, Styring S. Coupled activation of the donor and the acceptor side of photosystem II during photoactivation of the oxygen evolving cluster. Biochemistry. 1998;37(31):11039–11045. doi: 10.1021/bi980381h. [DOI] [PubMed] [Google Scholar]
- 33.Kapdan IK, Kargi F. Bio-hydrogen production from waste materials. Enzyme Microb Technol. 2006;38:569–582. [Google Scholar]
- 34.Marín-Navarro J, Esquivel MG, Moreno J. Hydrogen production by Chlamydomonas reinhardtii revisited: Rubisco as a biotechnological target. World J Microbiol Biotechnol. 2010;26:1785–1793. [Google Scholar]
- 35. Andersson B, Aro E-M (2001) Photodamage and D1 protein turnover in Photosystem II. Regulation of Photosynthesis, eds Aro E-M, Andersson B (Springer, Berlin), pp. 377–393.
- 36.Aro E-M, Virgin I, Andersson B. Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143(2):113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- 37.Vass I, Cser K. Janus-faced charge recombinations in photosystem II photoinhibition. Trends Plant Sci. 2009;14(4):200–205. doi: 10.1016/j.tplants.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Vass I, et al. Reversible and irreversible intermediates during photoinhibition of photosystem II: Stable reduced QA species promote chlorophyll triplet formation. Proc Natl Acad Sci USA. 1992;89(4):1408–1412. doi: 10.1073/pnas.89.4.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makarova VV, et al. Photoproduction of hydrogen by sulfur-deprived C. reinhardtii mutants with impaired photosystem II photochemical activity. Photosynth Res. 2007;94(1):79–89. doi: 10.1007/s11120-007-9219-4. [DOI] [PubMed] [Google Scholar]