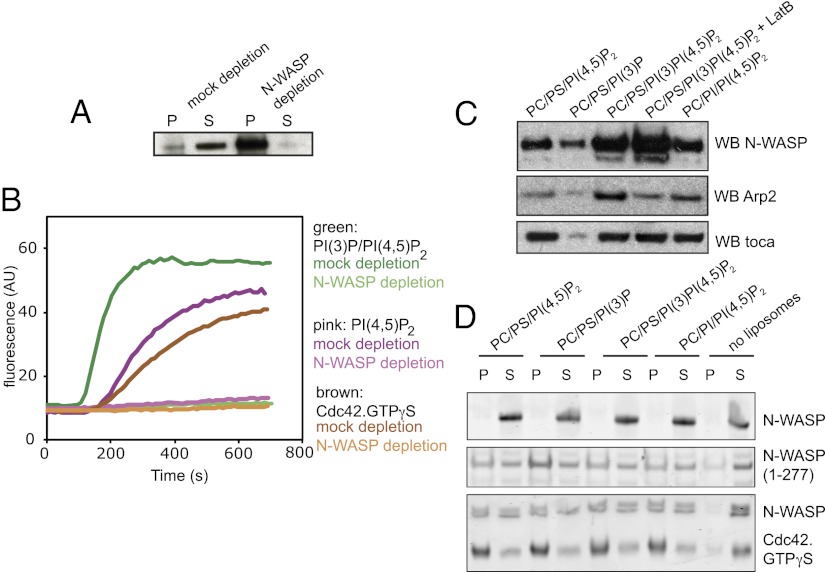
Fig. 5.
Actin polymerization stimulated by PI(3)P and PI(4,5)P2 uses N-WASP; however, the specificity for PI(3)P/PI(4,5)P2 binding is not within N-WASP itself. (A) Western blot of an immunodepletion showing the bead pellet and extract in the supernatant with control IgG and anti–N-WASP antibodies. (B) Pyrene actin assays on mock and N-WASP–depleted extract shows that N-WASP is critical for actin polymerization with Cdc42.GTPγS, PI(3)P, and PI(3)P/PI(4,5)P2 liposomes. (C) Liposome sedimentation assay from addition of liposomes into extract showing that although Arp2/3 complex and N-WASP binding to the liposomes is increased by the presence of PI(3)P, toca-1 binding is not. Use of latrunculin shows that N-WASP sedimentation is not due to bulk actin polymerization. Arp2 recruitment is driven by both membrane and polymerized actin. (D) Liposome sedimentation assays with the indicated lipid compositions with purified N-WASP, active N-WASP fragment (1–277), or N-WASP and activated Cdc42. There is no specificity in binding for PI(3)P/PI(4,5)P2-containing liposomes, suggesting an external recruitment factor. P, pellet; S, supernatant.