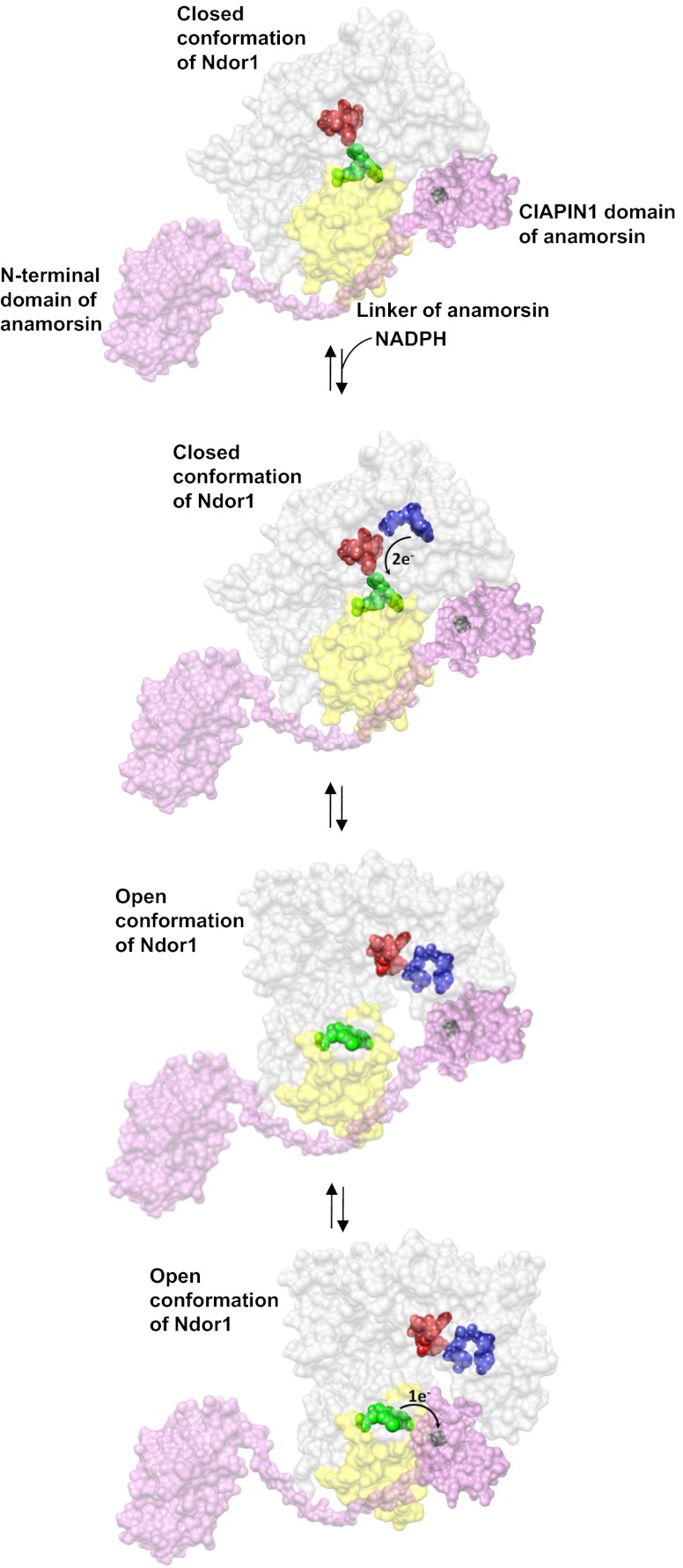
Fig. 5.
Model of the electron transfer process between Ndor1 and anamorsin. Anamorsin (in pink) can be tightly bound to both closed and open conformational states of Ndor1 (in gray) due to the specific recognition between an unstructured region of anamorsin and a region of the FMN-binding domain that is solvent exposed in both open and closed conformations (in yellow). The N-terminal domain of anamorsin is not involved in the protein–protein recognition process. Upon NADPH (in blue) binding, electrons can efficiently be transferred in the closed conformation of Ndor1 to FAD (in red) and then to FMN (in green) to produce the hydroquinone state of FMN. Interflavin electron transfer in Ndor1 significantly populates the open conformation, which exposes to the solvent the FMN moiety and allows the formation of a transient interaction with the [2Fe-2S] cluster region of anamorsin located in the CIAPIN1 domain. The latter interaction allows an efficient transfer of one electron from the hydroquinone state of FMN to the [2Fe-2S] cluster (in black).